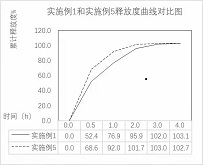
Atropine sulfate eye drops as well as preparation method and application thereof
The technology of atropine sulfate and eye drops is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and drug combinations, etc., and can solve the problems of the impact of patients' life and study, the short duration of drug efficacy, and the easy flow into the lacrimal sac for absorption, etc. Achieve the effects of increasing bioadhesion, reducing drug absorption, and simple and feasible preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
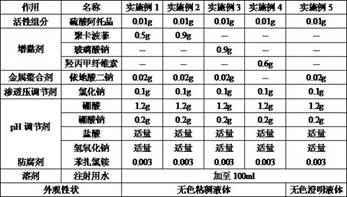
[0024] Table 1: Embodiment 1 ~ 4 eye drops formulation table
[0025]
Embodiment 1
[0026] Embodiment 1 preparation process
[0027] Step 1) Take an appropriate amount of water for injection to dissolve the prescribed amount of polycarbophil, stir to make the swelling complete, and sterilize at 121°C for 15 minutes for later use;
[0028] Step 2) Take another appropriate amount of water for injection to dissolve edetate disodium, boric acid, sodium borate, sodium chloride, benzalkonium chloride, add atropine sulfate to dissolve, filter, then add to the excipient solution obtained in step 1) and mix well; Use hydrochloric acid or sodium hydroxide to adjust the pH value to 3.5~6.5;
[0029] Step 3) Add water for injection to make up the volume so that the concentration of atropine sulfate in the eye drops is 0.01 mg / ml, and pack it separately to obtain the product. The obtained eye drops have a pH value of 3.5-6.0, an osmolality of 321 mOsm / kg, and a viscosity of 6800 cps.
Embodiment 2
[0030] Embodiment 2 preparation technology
[0031] Step 1) Take an appropriate amount of water for injection to dissolve the prescribed amount of polycarbophil, stir to make the swelling complete, and sterilize at 121°C for 15 minutes for later use;
[0032] Step 2) Take another appropriate amount of water for injection to dissolve edetate disodium, boric acid, sodium borate, sodium chloride, benzalkonium chloride, add atropine sulfate to dissolve, filter, then add to the excipient solution obtained in step 1) and mix well; Use hydrochloric acid or sodium hydroxide to adjust the pH value to 3.5~6.0;
[0033] Step 3) Add water for injection to make up the volume so that the concentration of atropine sulfate in the eye drops is 0.01 mg / ml, and pack it separately to obtain the product. The obtained eye drops have a pH value of 3.5-6.0, an osmolality of 298 mOsm / kg, and a viscosity of 7200 cps.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com