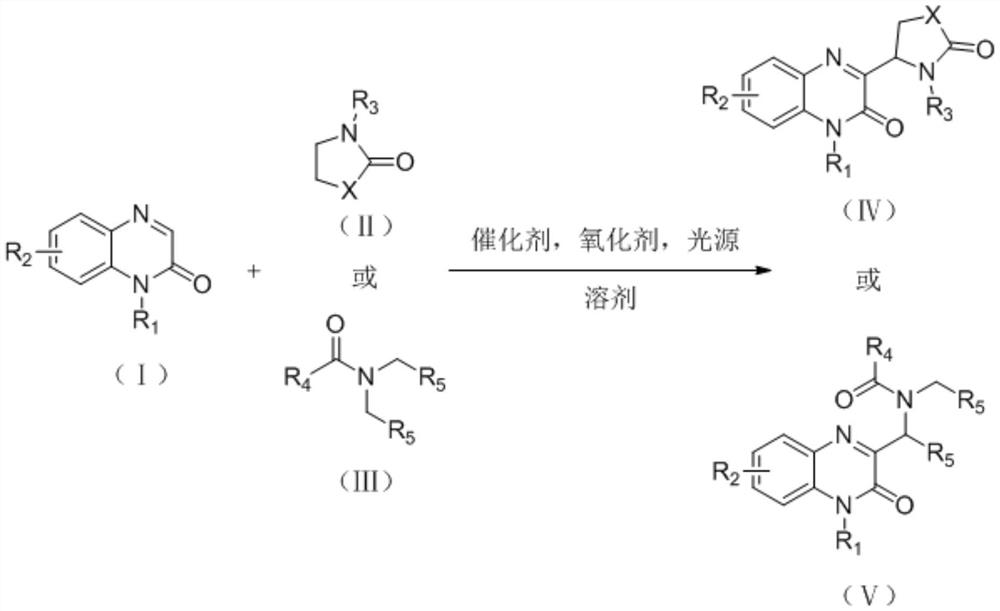
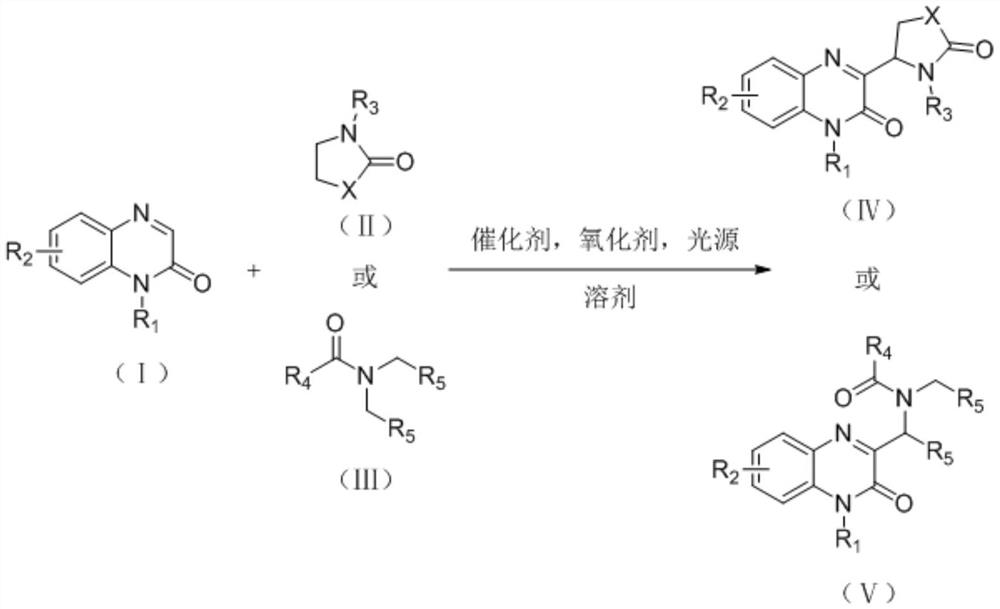
Synthesis method of 3-gamma-lactam/amide alkyl substituted quinoxalinone derivative
A quinoxalinone and amidoalkyl technology, which is applied in the field of synthesis of 3-γ-lactam/amidoalkyl substituted quinoxalinone derivatives, can solve the problem of increasing post-processing difficulty, high reaction temperature, and substrates. Scope limitation and other issues, to achieve the effect of being suitable for popularization and application, simple reaction operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
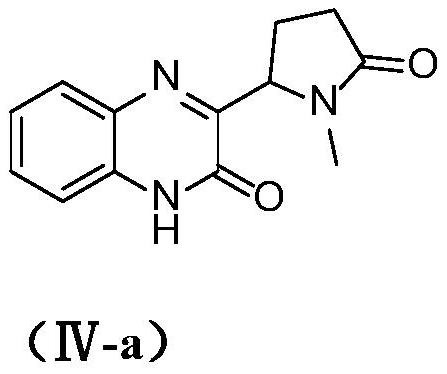
[0021] Example 1 3-(1-methyl-5-oxopyrrolidin-2-yl)quinoxalin-2(1H)-one (IV-a)
[0022]
[0023] Add compound (I) quinoxalin-2-one (58.4mg, 0.4mmol), potassium persulfate (27mg, 0.1mmol), 1-methylpyrrolidone (396mg, 4mmol) in a three-necked flask equipped with magnetic stirring, Water (1.5 mL) was added to the mixture, the mixture was exposed to the air and irradiated under 3W blue light, stirred and reacted at 25°C for 19 hours, the reaction solution was washed with saturated brine, the mixture was extracted with ethyl acetate, and the combined organic Anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified on a silica gel column using dichloromethane / methanol at a volume ratio of 50:1 to obtain the target product with a yield of 62% and an HPLC purity of 98.6%. 1 H NMR (400MHz, DMSO-d 6 )δ12.51(s,1H),7.74(dd,J=8.2,1.4Hz,1H),7.55–7.51(m,1H),7.33–7.28(m,2H),5.05(dd,J=9.0, 3.0Hz,1H),2.71(s,3H),2.46–2.34(m,1H),2.32–2.20(m,2H),1.99–1....
Embodiment 2
[0024] Example 2 6,7-Dimethyl-3-(1-methyl-5-oxopyrrolidin-2-yl)quinoxalin-2(1H)-one (IV-b)
[0025]
[0026] Add compound (I) 6,7-dimethylquinoxalin-2-one (69.6mg, 0.4mmol), potassium persulfate (54mg, 0.2mmol), 1-methyl Pyrrolidone (198mg, 2mmol), acetonitrile (1.5mL) was added to the mixture, the mixture was exposed to air and irradiated under 3W white light, stirred and reacted at 25°C for 18 hours, the reaction solution was washed with saturated brine, and the mixture was washed with acetic acid Ethyl ether extraction, combined organic layer with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified on a silica gel column using dichloromethane / methanol at a volume ratio of 60:1 to obtain the target product with a yield of 55% and an HPLC purity of 98.8%.
[0027] 1 H NMR (400MHz, DMSO-d 6 )δ12.38(s,1H),7.52(s,1H),7.07(s,1H),5.02(dd,J=8.8,3.2Hz,1H),2.69(s,3H),2.46–2.34(m ,1H),2.30(s,3H),2.27(s,3H),2.24–2.17(m,2H),1.93–1.86(m...
Embodiment 3
[0028] Example 3 1-methyl-3-(1-methyl-5-oxopyrrolidin-2-yl)quinoxalin-2(1H)-one (IV-c) (a:b=100:3, Crude product was detected by NMR)
[0029]
[0030]Add compound (I) 1-methylquinoxalin-2-one (64mg, 0.4mmol), ammonium persulfate (22.8mg, 0.1mmol), 1-methylpyrrolidone (99mg , 1mmol), dichloromethane (1.5mL) was added to the mixture, the mixture was exposed to the air and irradiated under 3W green light, stirred and reacted at 25°C for 20 hours, the reaction solution was washed with saturated brine, and the mixture was washed with acetic acid Ethyl ether extraction, combined organic layer with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The crude product was purified on a silica gel column using n-hexane / ethyl acetate at a volume ratio of 1:1 to obtain the target product with a yield of 79.5% and an HPLC purity of 97.8%. 1 H NMR (400MHz, CDCl 3 )δ7.84(d,J=8.0Hz,1H),7.57(t,J=7.8Hz,1H),7.37–7.32(m,2H),5.16(dd,J=8.6,2.6Hz,1H), 3.70(s,3H),2.84(s,3H),2.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com