Human hematopoiesis for oral administration or injection
A technology of essential amino acids and vitamins, applied in artificial blood for oral or injectable use. field, can solve the problem of not being able to improve the hematopoietic ability of the present application, and achieve the effects of improving oxygen-carrying ability, inhibiting virus activity, and being easy to preserve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
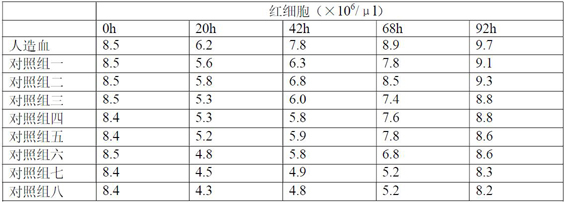
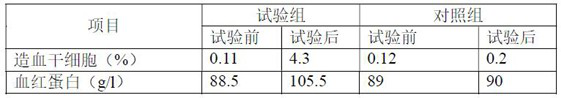
[0030] The artificial blood of the present invention is oral or injection dual-purpose artificial blood that can be absorbed through the intestinal tract, and its main components are composed of essential amino acids, trace elements, vitamins, nutrients and growth factors, specifically the following components:
[0031] Essential amino acids: methionine <0.0030g / 100g, glycine 0.0048g / 100g, glutamic acid 0.018g / 100g, leucine 0.0032g / 100g, serine 0.0055g / 100g, valine 0.0036g / 100g, isoleucine 0.0018g / 100g, histidine 0.0018g / 100g;
[0032] Trace elements: vanadium 0.00260mg / 100g, manganese 0.0706mg / 100g, iron 6.60mg / 100g, cobalt 0.00106mg / 100g, nickel<0.05mg / 100g, copper<0.02mg / 100g, lithium<0.02mg / 100g, arsenic 0.00724 mg / 100g, Selenium<0.003mg / 100g, Rubidium 0.0434mg / 100g, Molybdenum<0.003mg / 100g;
[0033] Nutrients: total flavonoids 7.88mg / 100g, folic acid <0.0015g / 100g, triterpenes, calculated as oleanolic acid, <2.61g / 100g, fructooligosaccharides 0.103mg / 100mL, β-carotene <1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com