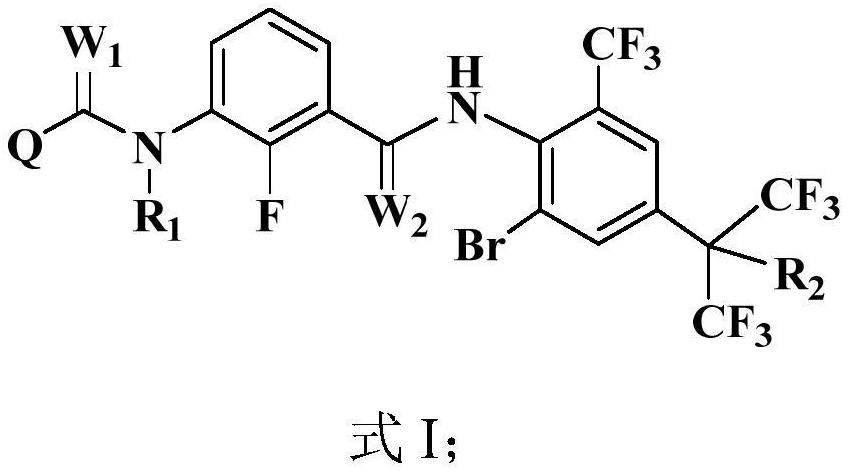
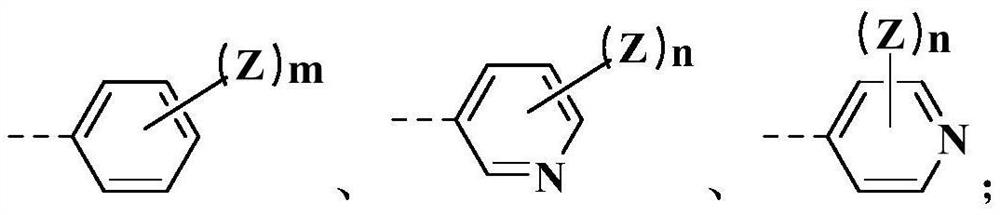
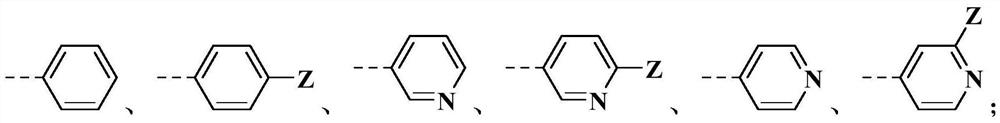
M-diamide compound and application thereof
A technology of meta-diamide and compound, applied in the field of meta-diamide compounds, can solve the problems of poor quick-acting, poor insecticidal effect, restricted promotion and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0158] This example provides a kind of intermediate diamide compound, that is, N-{3-[(2-bromo-4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl)- Preparation of 6-trifluoromethylphenyl)carbamoyl]-2-fluorophenyl}-N-ethylbenzamide (compound 1), the structure is as follows:
[0159]
[0160] The preparation method comprises the following steps:
[0161] (1) Synthesis of 3-ethylamino-2-fluorobenzoic acid methyl ester
[0162]
[0163] Dissolve methyl 2-fluoro-3-aminobenzoate (5.00g, 29.56mmol) in N,N-dimethylformamide (50mL), add bromoethane (6.44g, 59.12mmol) and potassium carbonate in sequence (12.26g, 88.68mmol), reflux for 16h. Thin-layer chromatography (TLC) monitored that when the reaction no longer proceeded, the reaction was terminated. Cool the reaction solution to room temperature, add ethyl acetate (200mL), water (100mL), extract and separate the layers, take the organic layer, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, concentrate un...
Embodiment 2
[0180] This example provides an intermediate diamide compound, namely N-[2-bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-trifluoro Preparation of methylphenyl]-3-[N-(ethyl)-2-chloro-isonicotinamide]-2-fluorobenzamide (compound 46) with the following structure:
[0181]
[0182] The preparation method comprises the following steps:
[0183] (1) N-[2-bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-trifluoromethylphenyl]-3-(ethyl Synthesis of (amino)-2-fluorobenzamide
[0184]
[0185] 3-Amino-N-[2-bromo-4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl)-6-trifluoromethylphenyl]-2- Fluorobenzamide (3.00g, 5.55mmol) was dissolved in methanol (50mL), acetaldehyde (610mg, 5.50mmol) and acetic acid (2.0g, 33.0mmol) were added successively, stirred for 4 hours under ice bath, and cyano Sodium borohydride (1.0 g, 16.5 mmol). When TLC monitors that the reaction no longer proceeds, the reaction is terminated. Add saturated aqueous sodium bicarbonate solution to the reaction solution, ad...
Embodiment 3
[0195] This example provides an intermediate diamide compound, namely N-[2-bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-trifluoro Preparation of methylphenyl]-3-[N-(n-butyl)-benzamido]-2-fluorobenzamide (compound 82) with the following structure:
[0196]
[0197] The preparation method comprises the following steps:
[0198] (1) N-[2-bromo-4-(1,1,1,2,3,3,3-heptafluoroprop-2-yl)-6-trifluoromethylphenyl]-3-(n Synthesis of Butylamino)-2-Fluorobenzamide
[0199]
[0200] 3-Amino-N-[2-bromo-4-(1,1,1,2,3,3,3-heptafluoropropan-2-yl)-6-trifluoromethylphenyl]-2- Fluorobenzamide (1.82g, 3.30mmol) was dissolved in 1,2-dichloroethane (30mL), and n-butyraldehyde (264mg, 3.60mmol) and acetic acid (1.2g, 20.0mmol) were added successively, and stirred under ice bath After 2 hours, sodium triacetoxyborohydride (1.4 g, 6.60 mmol) was added in portions. When TLC monitors that the reaction no longer proceeds, the reaction is terminated. Add saturated aqueous sodium bicarbonate solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com