COVID-19 subunit vaccine as well as preparation method and application thereof
A multivalent vaccine and vaccine composition technology, applied in the field of COVID-19 subunit vaccine and its preparation, can solve the problems of weakened effect and ineffectiveness that cannot be ruled out, and achieve the effects of stable and controllable quality, increased solubility, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: Preparation, identification and content determination of fusion protein HD-R1
[0099] According to NCBI (https: / / www.ncbi.nlm.nih.gov) the gene sequence of the RBD neutralizing epitope (artificial Synthetic, the amino acid sequence is as shown in SEQ ID No.1, which is the 319-593th position of the S protein amino acid sequence), and the gene sequence of the Fc protein in human immunoglobulin IgG (artificially synthesized, the nucleotide sequence is as in SEQ ID No. .32, the amino acid sequence is as shown in SEQ ID No.33), and the fusion protein HD-R1 (nucleotide sequence is as shown in SEQ ID No.2, and amino acid sequence is as shown in SEQ ID No.3) is prepared by genetic engineering means ).
[0100] (1) Construction and identification of PCDNA3.1-HD-R1 recombinant expression plasmid
[0101] The double-stranded DNA molecule HD-R1 was inserted between the BamHI and XhoI restriction sites of the plasmid vector pcDNA3.1, and the recombinant expression plas...
Embodiment 2
[0108] Embodiment 2: Preparation, identification and content determination of other fusion proteins
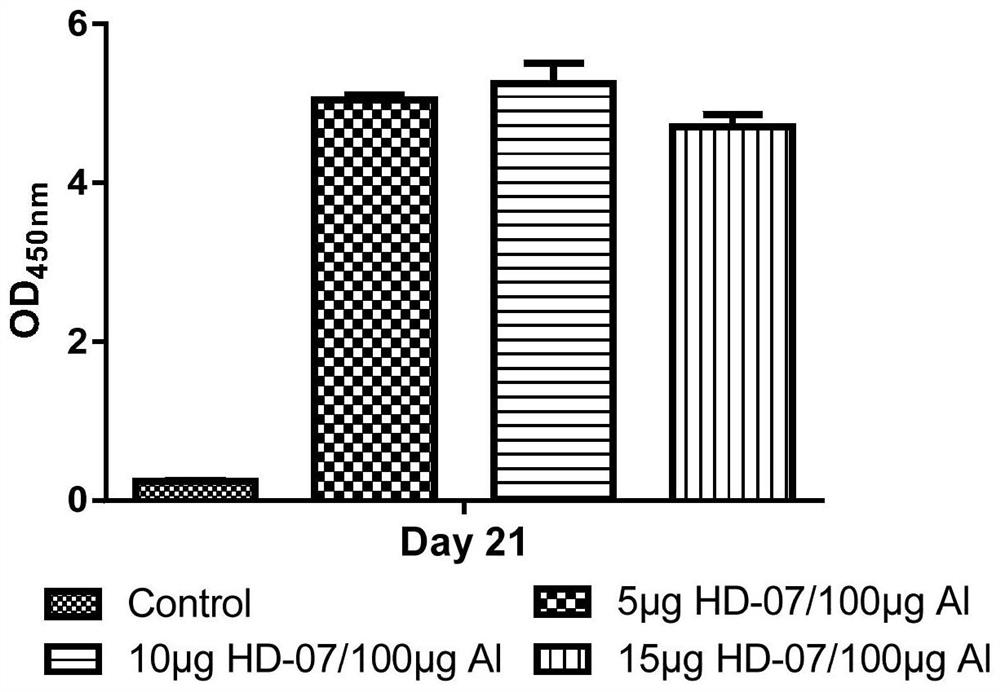
[0109] HD-R2, HD-05, HD-R3, HD-01, HD-02, HD-03, HD-04, HD-06, HD-07, HD-08, HD-09, HD-10 in Table 2 , HD-11, HD-12, HD-S1 and HD-S and other 16 kinds of fusion protein preparation, identification and content determination, except that the corresponding plasmid vector pcDNA3.1 contains the 16 kinds of fusion proteins corresponding to Table 2 Except for the difference in the nucleotide sequence relative to the amino acid sequence, the steps of its preparation, identification and content determination are similar to those in Example 1. Among them, 16 kinds of fusion proteins are obtained by linking different RBDs with corresponding tags through linkers.
[0110] Table 2: Sources and characteristic information of 17 fusion proteins
[0111]
[0112]
Embodiment 3
[0113] Example 3: Preparation of subunit vaccine and mouse immunization
[0114] (1) Dilute the fusion proteins HD-R1, HD-R2, HD-S1 and HD-S prepared in Example 1 and Example 2 with PBS buffered saline solution, and add aluminum adjuvant (Al) to fully emulsify the mixture The corresponding subunit vaccine can be obtained, and the ratio of fusion protein and aluminum adjuvant is shown in Table 3.
[0115] Table 3: Subunit vaccine ratio
[0116]
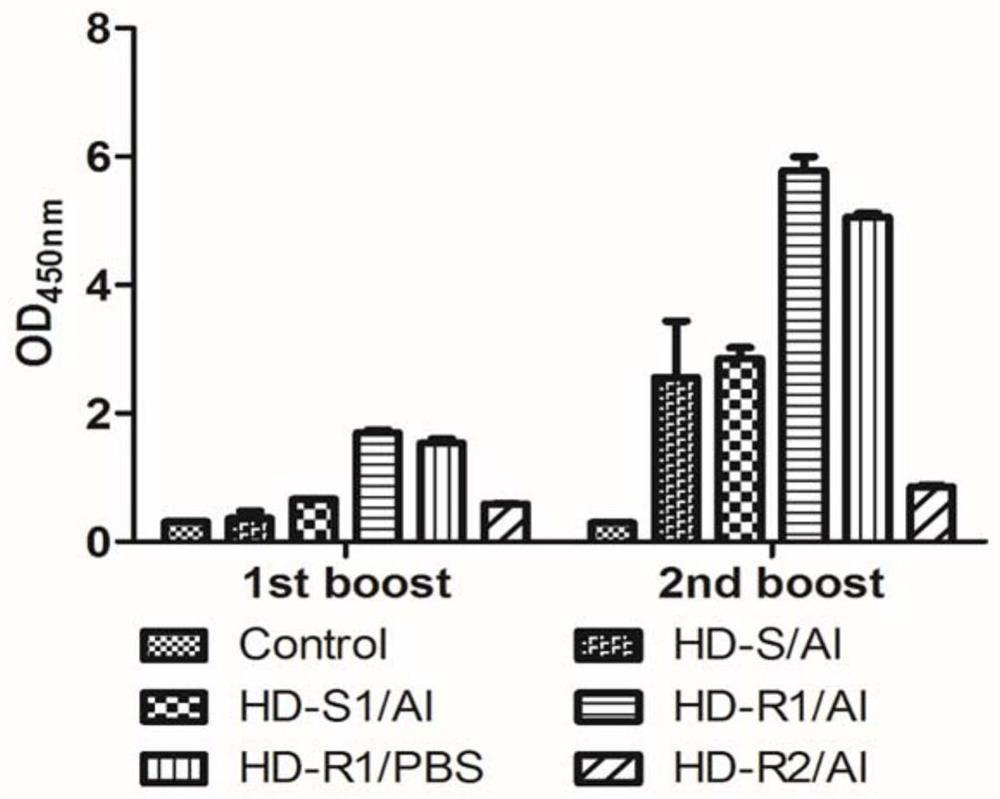
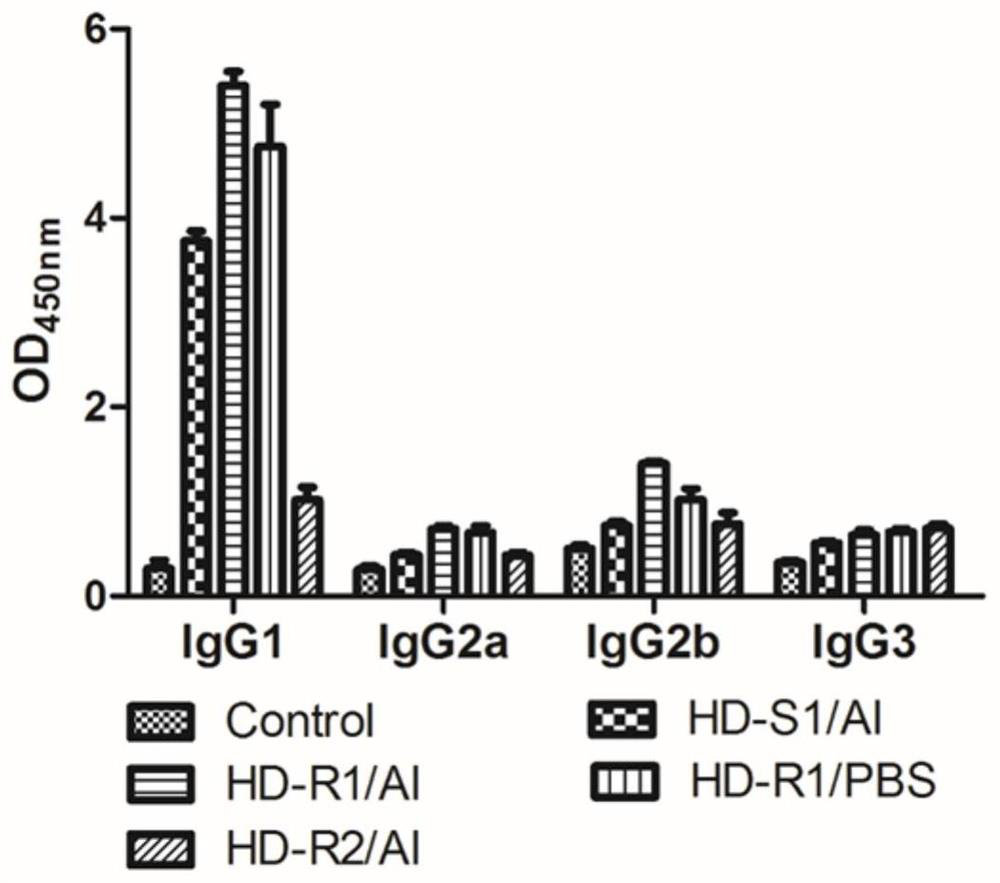
[0117] (2) For the fusion protein vaccine group and the control group above, the experiment adopted an initial immunization (1st boost) and a second boost (2nd boost) immunization scheme, and the mice were intramuscularly injected with the prepared vaccine on the 0th and 30th days, each The volume of each injection was 10 μg / 100 μL, and the control group was injected with the same volume of PBS solution; blood was collected on the 30th and 40th days, and 0.1 mL to 0.2 mL of blood was collected from each mouse, placed at 0 ° C for 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com