Method for efficiently fermenting and producing rhCG by using CHO cells
A fermentation method and cell technology, applied in the field of protein and cell culture, can solve the problems of long culture time, time-consuming, low research and production efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Cells, culture medium and other reagents used in the fermentation of embodiment 1
[0019] The CHO cells used for the fermentation were self-made by the applicant. LipofectamineTM2000 liposome transfection reagent was used to transfect the vector carrying rhCGα and β subunits into the CHO cell K1 strain. The cells were subsequently acclimatized without serum and had been in the applicant’s rhCG It has been used in pilot production for more than 18 months.
[0020] The medium formulated by the applicant for the fermentation process:
[0021] Subculture medium:
[0022] CD CHO AGT 24.3mg / ml, dihydrogen phosphate monohydrate 2.69mg / ml, disodium hydrogen phosphate 4.33mg / ml, L-arginine 550mg / ml, L-asparagine 1300mg / ml, L-aspartic acid Acid 400mg / ml;
[0023] Fermentation mixed medium:
[0024] CD CHO AGT 24.3mg / ml, CD OPTICHO AGT 9.66mg / ml, sodium dihydrogen phosphate monohydrate 2.69mg / ml, disodium hydrogen phosphate 4.33mg / ml, biotin 0.15mg / ml, folic acid 11.5mg / ml, m...
Embodiment 2
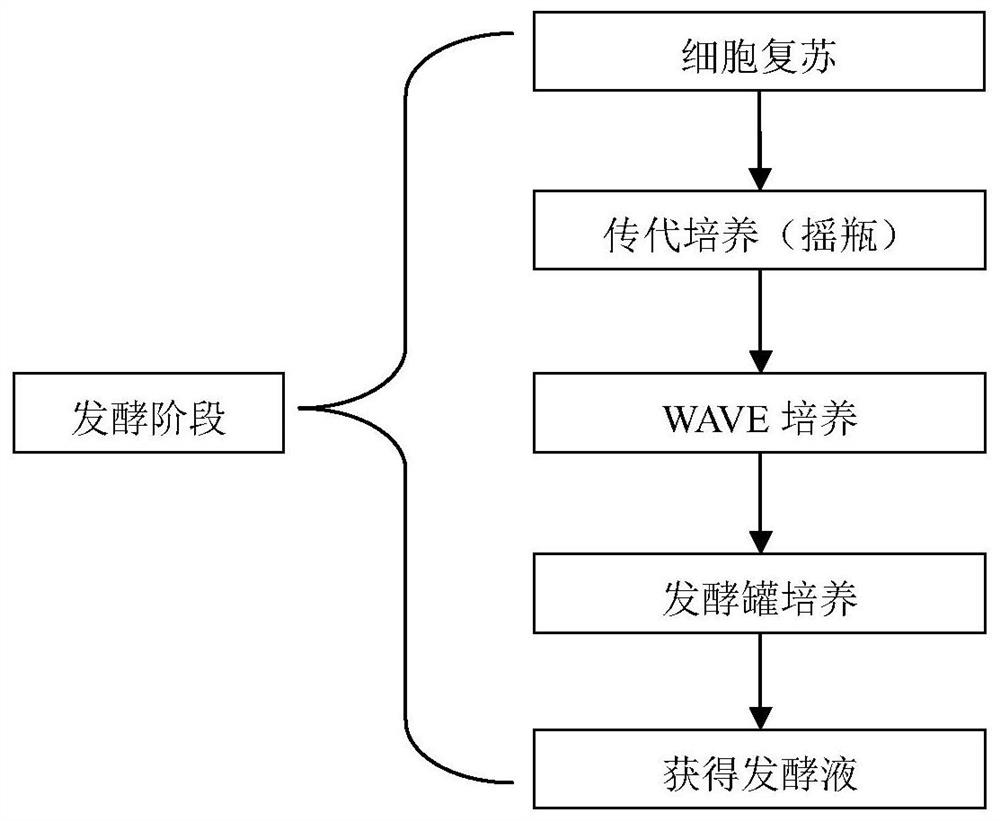
[0035] Embodiment 2 basic fermentation production process
[0037] 1) Take the cell cryopreservation tube and place it in a 37°C water bath until the frozen cell suspension melts.
[0038] 2) Transfer the frozen cells to a centrifuge tube containing 6-7ml of CHO subculture medium, centrifuge at 1000rpm for 5 minutes, and remove the supernatant.
[0039] 3) Pipette 10ml of CHO subculture medium in portions and gently blow the clumped cells at the bottom of the centrifuge tube.
[0040] 4) Inoculate into a 125ml shake flask containing 10ml of CHO subculture medium, so that the final volume is about 20ml, and the seeding cell density is 0.4-1.0*10 6 cells / ml. Place the shake flask in a carbon dioxide incubator at 36.5±1°C, 5±3% CO 2 , 125±10rpm for cultivation.
[0041] pass on:
[0042] 1) Shake flask culture: place the cell shake flask in a carbon dioxide incubator at 36.5±1°C, 5±3% CO 2 , 125±10rpm for cultivation. Count the cells every day, whe...
Embodiment 3
[0047] The optimization of embodiment 3 fermentation process
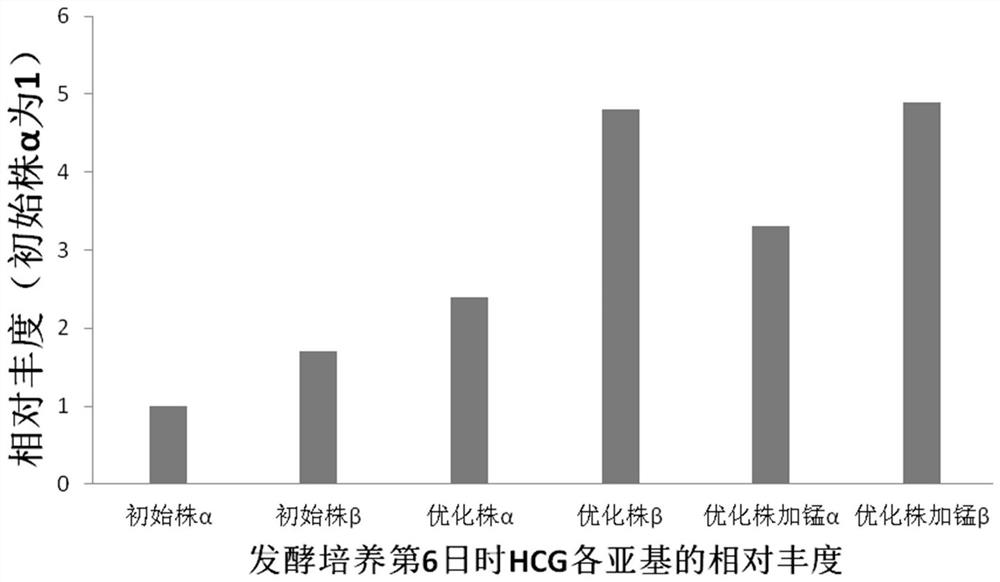
[0048]After optimizing the fermentation parameters and cells and other factors in the early stage, our method achieved a recombinant protein expression level of about 180 mg / L within 12 days. In order to further increase yield / lower cost at similar yield, we have made further improvements to our custom media. With reference to the prior art, try to add MnCl to the culture medium 2 (3mg / ml is added in the fermentation mixed culture medium), hydrocortisone, sodium pyrophosphate, the result is as follows:
[0049] Table 1 Effect of adding manganese ions on expression level in optimized cell line and fermentation mixed medium (average value of three tank samples)
[0050]
[0051] Further research on the transcript level results as figure 2 Shown: We found that the ratio of β subunit to α subunit in the optimized cell line was significantly higher than that of the initial cell line, which means that the transcri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com