A kind of 2-thioquinazolone compound and its preparation method and application
A technology for thioquinazolones and compounds, applied in the field of 2-thioquinazolones and their preparation, capable of solving problems such as cumbersome operations and complicated process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
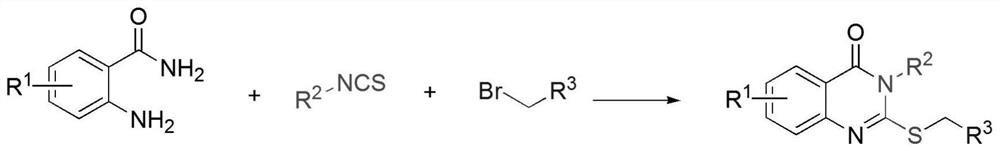
[0031] The invention provides a kind of preparation method of 2-thioquinazolone compound, it is characterized in that, comprises the following steps:
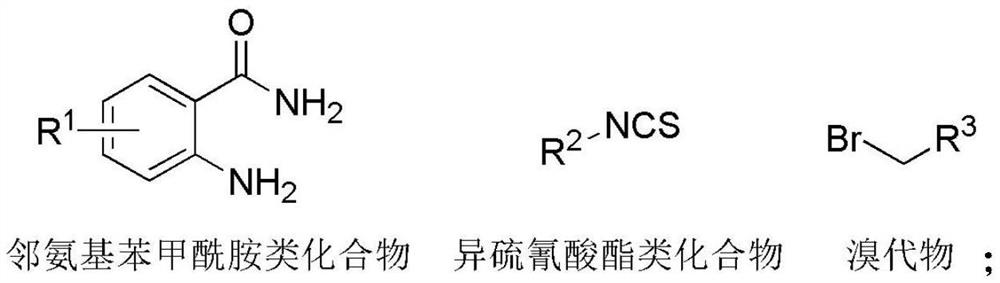
[0032] Mix anthranilamide, isothiocyanate compound, bromide and polar organic solvent, and carry out a series cyclization reaction under air atmosphere to obtain 2-thioquinazolinone compound;
[0033]
[0034] Among them, R 1 includes hydrogen, alkyl, alkoxy, halogen or heteroatom;
[0035] R 2 Including phenyl, naphthyl, benzoyl, alkyl substituted phenyl, alkoxy substituted phenyl, halogen substituted phenyl, haloalkyl substituted phenyl, nitro substituted phenyl, nitrile substituted phenyl, alkyl, Cycloalkyl or ester groups;
[0036] R 3 Including ester, phenyl, alkenyl or alkynyl.
[0037] In the present invention, unless otherwise specified, all raw material components are commercially available products well known to those skilled in the art.
[0038] In the present invention, the anthranilamide compounds include ...
Embodiment 1
[0057] Add 0.15 mmol of 2-amino-3-methylbenzamide, 0.1 mmol of phenyl isothiocyanate, 0.3 mmol of ethyl bromoacetate, 1.0 mL of acetonitrile into the reaction tube, heat to 80°C, and stir for 12 hours. After the reaction, it was separated and purified by column chromatography, the eluent was petroleum ether and ethyl acetate, and the volume ratio of petroleum ether and ethyl acetate in the eluent was 5:1, and the compound having the formula I-1 was obtained. Structured 2-thioquinazolinones (70% yield, 99.9% purity).
[0058] The structure of the obtained product is characterized, and the structural characterization data are as follows:
[0059] 1 H NMR (400MHz, CDCl 3 ,ppm)δ8.10-8.08(d,J=8Hz,1H),7.59-7.55(m,4H),7.38-7.36(q,J=4Hz,2H),7.32-7.28(t,J=8Hz, 1H), 4.24-4.18 (q. J = 8Hz, 2H), 3.89 (s, 2H), 2.57 (s, 3H), 1.30-1.26 (t, J = 8Hz, 3H).
[0060] 13 C{ 1 H}NMR (100MHz, CDCl 3 , ppm) δ168.5, 162.0, 154.8, 146.1, 135.7, 135.3, 134.8, 130.2, 129.8, 129.2, 125.7, 124.9, 119...
Embodiment 2
[0063] Add 0.15 mmol of 2-amino-4-methylbenzamide, 0.1 mmol of phenylisothiocyanate, 0.3 mmol of ethyl bromoacetate, and 1.0 mL of acetonitrile into the reaction tube, heat to 80°C, and stir for 12 hours. After the reaction is finished, it is separated and purified by column chromatography, and the eluent is petroleum ether and ethyl acetate, and the volume ratio of petroleum ether and ethyl acetate in the eluent is 5:1, and the compound having the formula I-2 is obtained. Structured 2-thioquinazolinones (yield 68%, purity 99.9%).
[0064] The structure of the obtained product is characterized, and the structural characterization data are as follows:
[0065] 1 H NMR (400MHz, CDCl 3 , ppm) δ8.12-8.10(d, J=8Hz, 1H), 7.56-7.54(t, J=8Hz, 3H), 7.37-7.35(q, J=4Hz, 3H), 7.23-7.21(d, J=8Hz, 1H), 4.25-4.20 (q, J=8Hz, 2H), 3.88(s, 2H), 1.32-1.28 (t, J=8Hz, 3H).
[0066] 13 C{ 1 H}NMR (100MHz, CDCl 3 , ppm) δ168.7, 161.7, 155.9, 147.6, 145.7, 135.7, 130.1, 129.8, 129.2, 127.7, 127...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com