Preparation method of chloroalkoxy-1, 3-diphenyl-beta-diketone
A chloroalkoxy, methoxyphenyl technology, applied in the field of organic synthesis, can solve the problems of catalyst residue, unstable and easy to decompose, difficult to purify, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
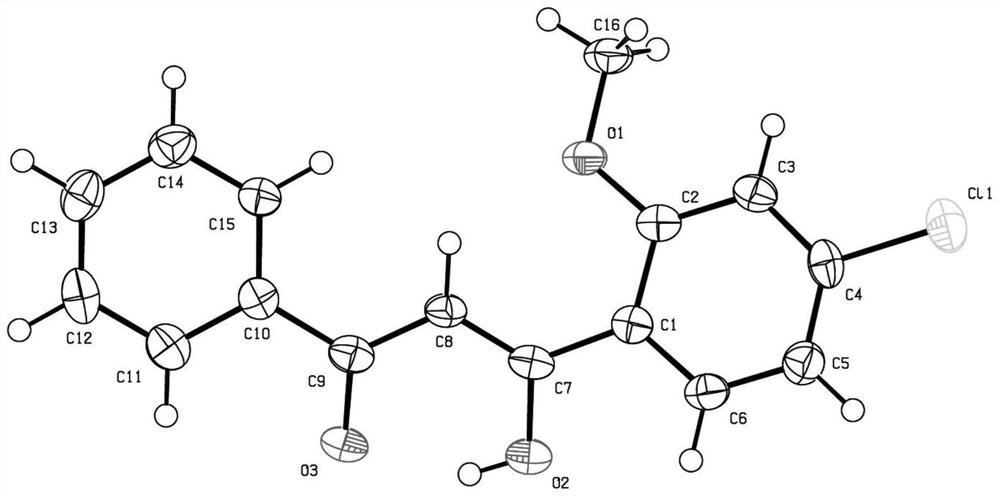
[0031] Preparation of 1-(3-chloro-4-methoxyphenyl)-3-phenyl-1,3-propanedione, its structural formula is as follows:
[0032]
[0033] Add 40 mL of dry N,N-dimethylformamide, 4.10 g (20 mmol) of methyl 3,4-dichlorobenzoate, and 1.60 g of sodium hydride (60% solid paraffin mixture) into a 100 mL round bottom flask. (40mmol), stirred at room temperature for 10 minutes. At room temperature, 2.88 g (24 mmol) of acetophenone was added in 4 batches. After stirring for 30 minutes, the temperature was raised to 40° C. and stirring was continued for 3 hours. After cooling to room temperature, it was poured into 200 mL of ice water with stirring, acidified (neutralized with 36% hydrochloric acid) to weak acidity, and left to stand overnight. The above aqueous solution was poured off, and the viscous organic solid was recrystallized twice from a mixture of petroleum ether and ethyl acetate to obtain the target product with a yield of 75%. Its proton nuclear magnetic resonance spectru...
Embodiment 2
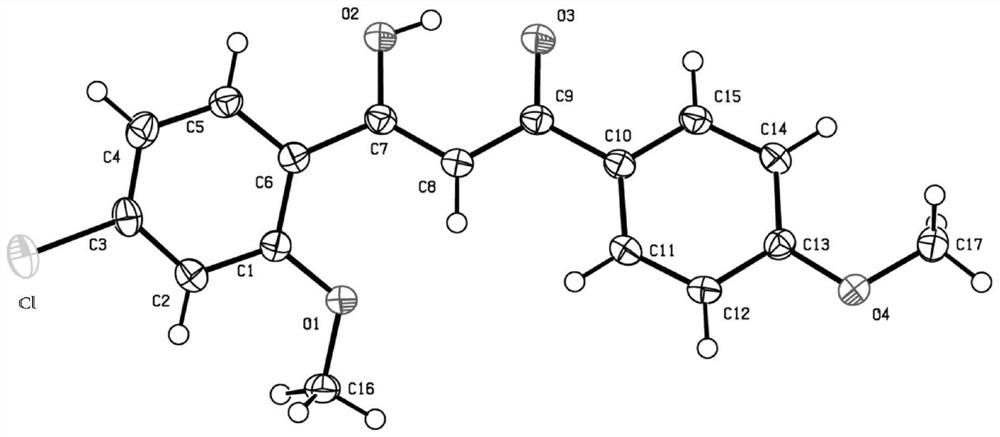
[0036] Preparation of 1-(3-chloro-4-methoxyphenyl)-3-(4-methoxyphenyl)-1,3-propanedione, its structural formula is as follows:
[0037]
[0038] The difference between this example and Example 1 is that the target product was prepared with 4-methoxyacetophenone instead of acetophenone, and the yield was 71%. Its proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum result are as follows: 1 H NMR (300MHz, CDCl 3 )δ:7.99-7.93(m,3H),7.86(dd,J 1 =8.4Hz,J 2 =2.4Hz,1H),6.99-6.95(m,3H),6.67(s,1H),3.96(s,3H),3.87(s,3H). 13 C NMR (75MHz, CDCl 3 )δ: 184.8, 183.3, 163.2, 158.1, 129.2, 129.0, 127.8, 127.3, 122.9, 114.0, 111.5, 91.6, 56.3, 55.5. DEPT135 (75MHz, CDCl 3 )δ: 129.2(CH), 127.3(CH), 114.0(CH), 111.5(CH), 91.6(CH), 56.3CH 3 ),55.5CH 3 ).
Embodiment 3
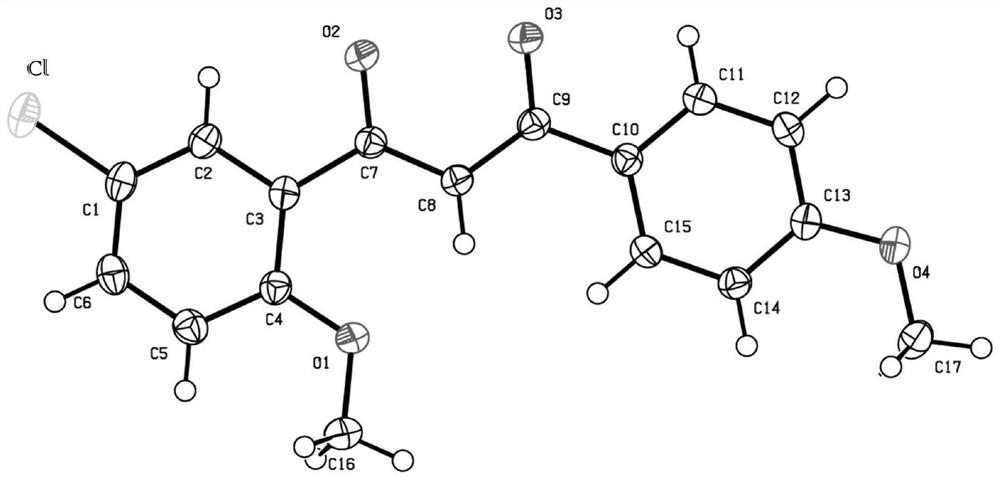
[0040] Preparation of 1-phenyl-3-(4-chloro-2-methoxyphenyl)-1,3-propanedione, its structural formula is as follows:
[0041]
[0042] Method 1: The difference between Method 1 of this example and Example 1 is that methyl 2,4-dichlorobenzoate is used instead of methyl 3,4-dichlorobenzoate to prepare the target product with a yield of 65%. Its proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum result are as follows: 1 HNMR (300MHz, CDCl 3 )δ:7.96-7.88(m,3H),7.54-7.47(m,3H),7.06(s,1H),7.03-6.98(m,2H),3.95(s,3H). 13 C NMR (75MHz, CDCl 3 )δ: 186.1, 182.8, 159.0, 138.9, 135.8, 132.3, 131.4, 128.6, 127.2, 123.4, 121.1, 112.3, 98.4, 56.1. DEPT135 (75MHz, CDCl 3 )δ: 132.4(CH), 131.4(CH), 128.6(CH), 127.2(CH), 121.2(CH), 112.4(CH), 98.4(CH), 56.1(CH 3 ).
[0043] Method 2: The difference between Method 2 of this example and Example 1 is that methyl 2-fluoro-4-chlorobenzoate is used instead of methyl 3,4-dichlorobenzoate to prepare the targe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com