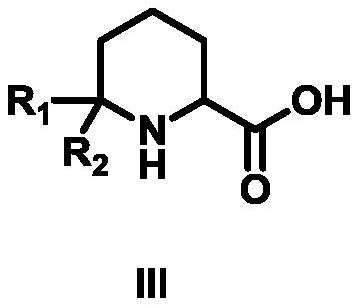
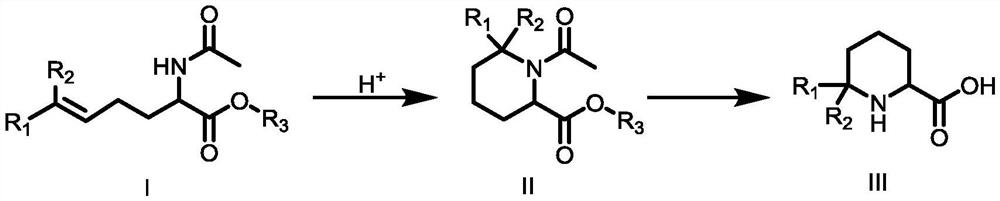
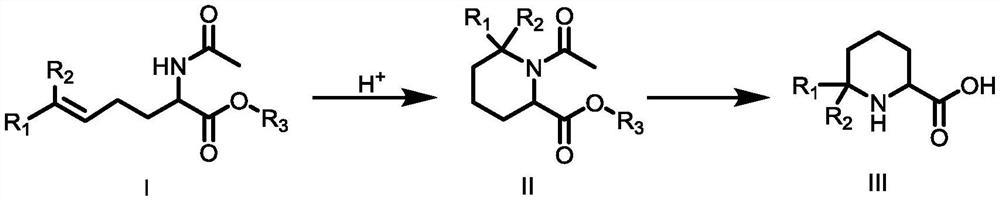
Synthetic method of 6,6-dialkylpiperidine-2-carboxylic acid compound
A synthetic method and compound technology, applied in the direction of organic chemistry, etc., to achieve the effect of avoiding by-products, less reaction by-products, and high economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step: the synthesis of compound 1-acetyl-6,6-dimethylpiperidine-2-carboxylic acid ethyl ester
[0031] 2-(Acetylamino)-6-methyl-5-heptanoic acid ethyl ester (10g, 44mmol, 1eq) was added to 100ml of trifluoroacetic acid, and the reaction was carried out at 50°C for 2 hours until TLC detection Reached the reaction end point, cooled to room temperature, added to ice water, adjusted the pH to 8-9 with sodium bicarbonate, extracted twice with 600 mL of ethyl acetate, combined the organic phases and backwashed with saturated brine, added anhydrous sodium sulfate to dry, It was distilled under reduced pressure and eluted by column chromatography. The eluent used in the column chromatography was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 5:1 to obtain 8.1 g of compound 1-acetyl-6,6- Dimethylpiperidine-2-carboxylate, 81.00% yield, 98% purity, 1 HNMR (500MHz, CDCl 3 )δ4.66(t,J=8.0Hz,1H,NCH),4.14(q,J=6.0Hz,2H,CH 2 CH 3 ),2.22(ddd,J=18...
Embodiment 2
[0038]Step (1) in Example 2 to Example 3 was carried out with an acid solution different from that in Example 1. In Example 2, glacial acetic acid was used for the reaction. Compared with Example 1, the yield decreased and was 70.00%. Especially, using hydrochloric acid to carry out the reaction in Example 3, not only the reaction rate is obviously slow, and more by-products are generated during the heating process, so that the reaction yield is significantly lower than that of Example 1, only 23.01%.
Embodiment 4
[0039] Step (1) in Example 4 to Example 5 adopts a reaction temperature different from that in Example 1. Compared with Example 1, Example 4 lowers the reaction temperature to 25°C, the reaction rate is significantly reduced, and the reaction yield within the same reaction time is reduced to only 9.30%; Example 5 raises the reaction temperature to 70°C, which promotes The occurrence of side reactions reduces the reaction yield to 75.00% compared to Example 1.
[0040] Step (2) in Example 6 to Example 7 adopts the sodium hydroxide of different dosage from Example 1 to react, and relative to Example 1, Example 6 and Example 7 reduce sodium hydroxide relative to Example 1 respectively. amount to 3 equivalents of ethyl 1-acetyl-6,6-dimethylpiperidine-2-carboxylate and increase the amount of sodium hydroxide to 1-acetyl-6,6-dimethylpiperidine- 5 equivalents of ethyl 2-carboxylate, the reaction yield is slightly lower than that of Example 1, which are 72.70% and 80.00%, respectivel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com