Recombinant escherichia coli for synthesizing glutathione and application of recombinant escherichia coli
A technology of recombinant Escherichia coli and glutathione, applied in the field of bioengineering, can solve the problems of high price of acetyl phosphate and low glutathione yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Connecting SpyCatcher / SpyTag to the N-terminus of GshF and the C-terminus of PPKJT to construct different recombinant expression plasmids and engineering bacteria
[0031] Select the PPKJT (amino acid sequence as shown in SEQ ID NO.4) screened by the laboratory and the GshF (amino acid sequence as shown in SEQ ID NO.2) of directed evolution, and respectively pass flexible linker(GGGGS) 2(nucleotide sequence as shown in SEQ ID NO.17: ggtggcggtg gctcgggcgg tggtgggtcg) connect SpyCatcher (amino acid sequence as shown in SEQ ID NO.1) and SpyTag (amino acid sequence as shown in SEQ ID NO.3) (SpyCatcher and SpyTag And the flexible linker was synthesized by Shanghai Bioengineering Co., Ltd.), CM3 was connected to the pET28a vector by homologous recombination, and PPKJT was synthesized by Shanghai Bioengineering Co., Ltd. Each gene has a separate promoter or RBS regulation, and the plasmid Transfer to BL21(DE3) respectively. The specific primer sequences are shown ...
Embodiment 2
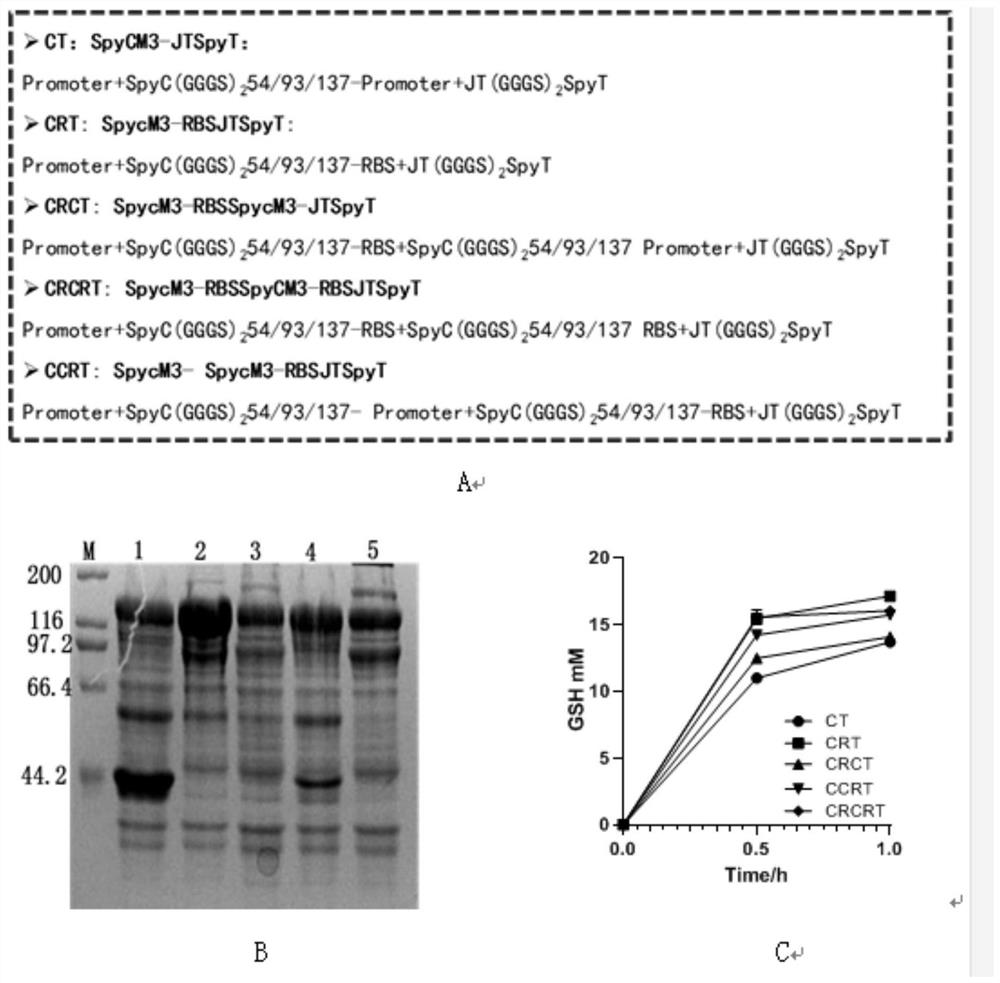
[0036] Example 2. Construction of recombinant expression plasmids optimized for assembly protein expression and engineering bacteria
[0037] Insert different gene copy numbers into the same plasmid and regulate the expression of assembled protein through promoter or RBS, construct five different plasmids by homologous recombination, and transfer the plasmids into BL21(DE3) respectively. The specific primer sequences are shown in Table 1, and the sequences were synthesized by Shanghai Bioengineering Co., Ltd.
[0038] Preparation of linearized vector: PCR amplification was performed with pET28a as template and pET28a-F and pET28a-R as upstream and downstream primers respectively. The reaction system is: template 2 μl; PrimerSTAR Max 25 μl; forward and reverse primers 20 mM each; add sterile water to a total volume of 50 μl. The reaction conditions were: pre-denaturation at 98°C for 5 min; denaturation at 98°C for 10 s; annealing at 60°C for 30 s; extension at 72°C for 4 min, ...
Embodiment 3
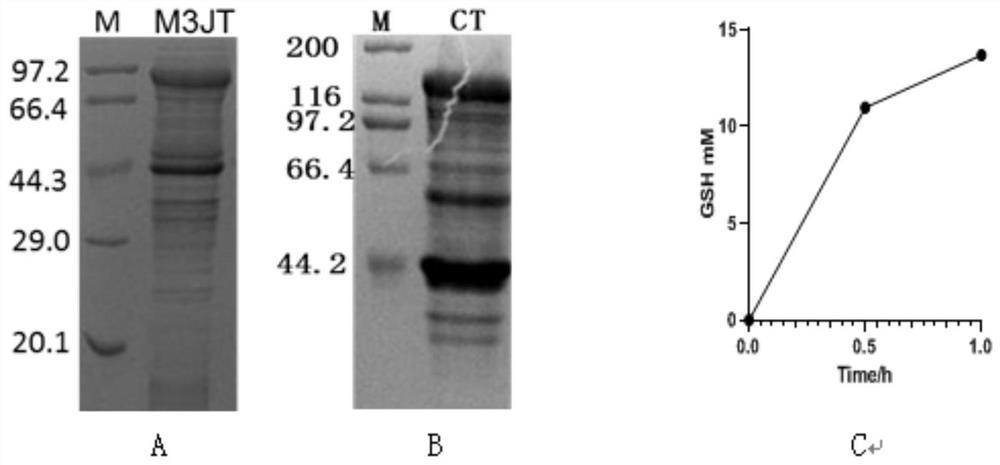
[0041] Example 3. Verification of CM3 and JTT intracellular self-assembly and ATP regeneration
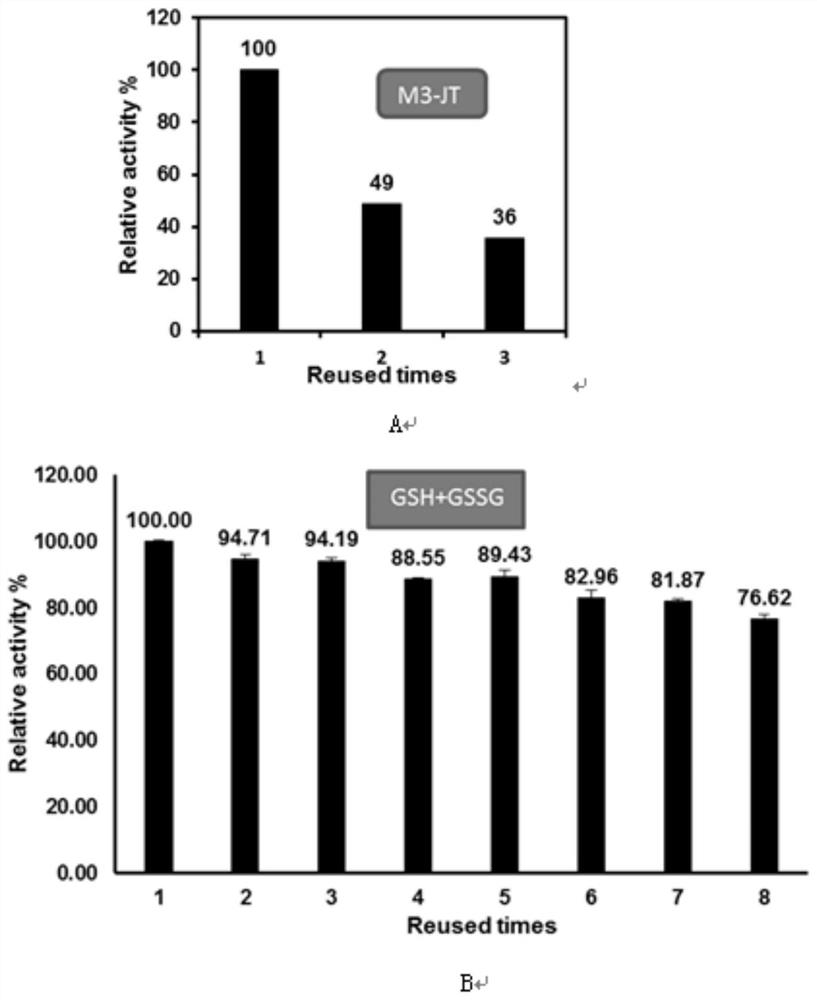
[0042] ATP is an essential cofactor in the production of GSH, so the regeneration of ATP is an important factor to reduce the cost of GSH production. Polyphosphate kinase PPK can use polyphosphate (PolyP) and ADP as substrates to achieve efficient regeneration of ATP. Compared with single-enzyme catalysis, the efficiency of intermediate transfer and cofactor regeneration can be improved by aggregating several enzymes involved in metabolic pathways in organisms to form a multi-enzyme complex, and at the same time, it can also reduce the feedback inhibition of products. GshF is intracellularly coupled to PPK to solve the problem of ATP regeneration during whole-cell catalyzed synthesis of GSH. In this study, it is hoped that SpyCatcher and SpyTag can realize dual-enzyme intracellular self-assembly and be used for ATP regeneration. The result is as figure 1 As shown in A and B, a l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com