Reagent and method for determining total amount of sesquiterpene lactone containing alpha, beta-unsaturated ketone in feverfew
A sesquiterpene lactone, unsaturated technique for use in pharmaceutical analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of 4-chloro-N-(2-mercaptoethyl)benzamide
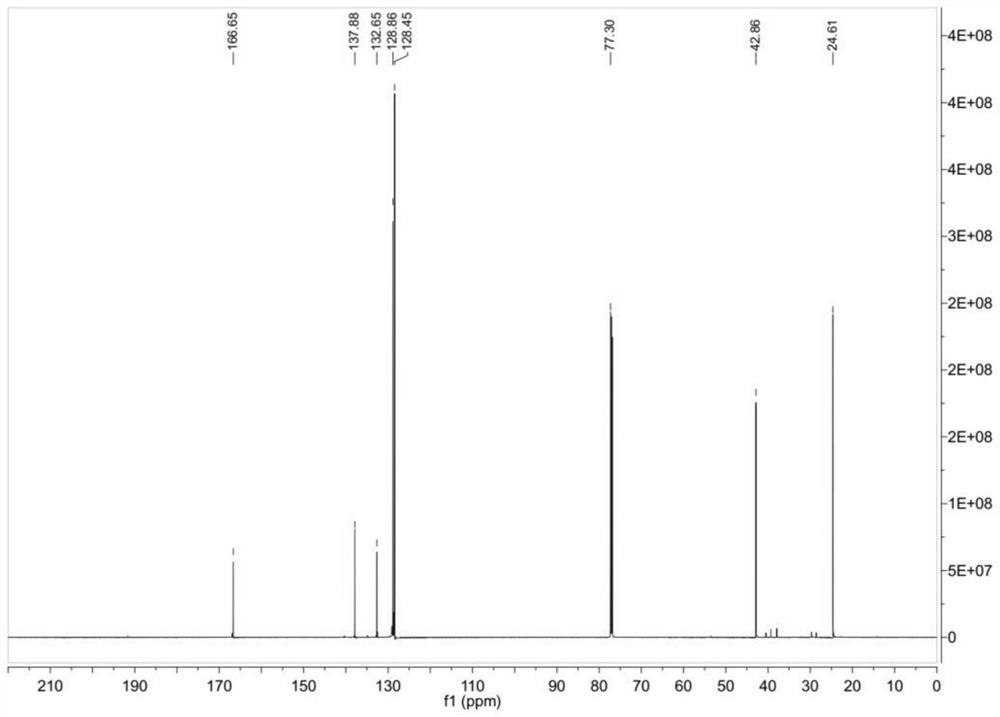
[0038] In dichloromethane (30ml) at 0°C, 4-chlorobenzoyl chloride (1.00g, 5.71mmol) was slowly added, followed by β-mercaptoethylamine (0.45g, 5.86mmol), and heated under reflux for 2 hours. The reaction solution was washed with saturated sodium bicarbonate (10mL) and concentrated brine (10mL) accordingly, Na 2 SO 4 Dry and concentrate under reduced pressure to a yellowish solid. After repeated crystallization from a mixed solvent of n-hexane-dichloromethane (1:1, v / v), a white solid (0.62 g, yield 50.3%) was obtained. ESI-MS: m / z 214.0087[M-H] - (calculated m / z 214.0093, C 9 h 9 ClNOS); 1 H NMR (600MHz, CDCl 3 )δ7.64 (2H, d, J = 8.6Hz), 7.31 (2H, d, J = 8.6Hz), 6.80 (1H, br s), 3.53 (2H, d, J = 6.4Hz), 2.69 (2H ,dt,J=8.4Hz,6.4Hz),1.38(H,t,J=8.4Hz); 13 C NMR (150MHz, CDCl3) δ166.6, 137.9, 132.6, 128.7, 128.4, 42.9, 24.6, among which, the hydrogen and carbon spectra are shown in figure 2 and image 3 .
Embodiment 2
[0040] The detection method of 4-chloro-N-(2-mercaptoethyl)benzamide
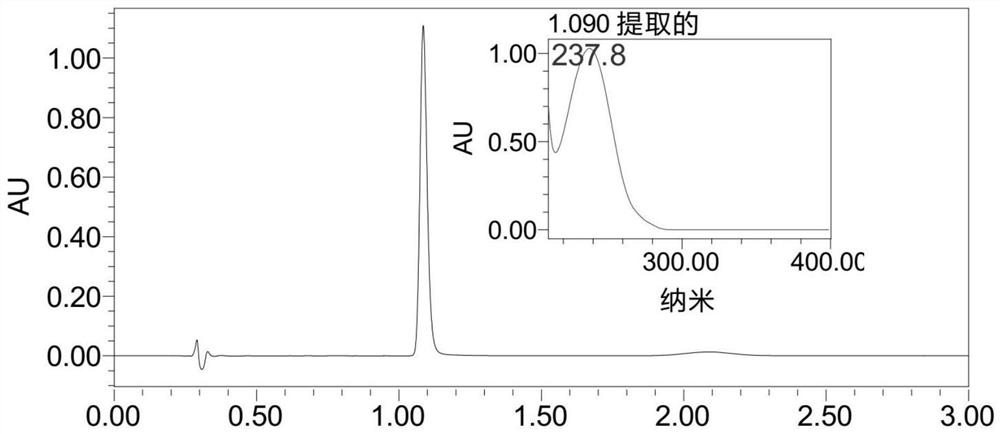
[0041] Using ACQUITYUPLC ultra-high performance liquid chromatography, the chromatographic conditions are chromatographic column Waters BEH C18 (50×2.1mm, 1.7μm), mobile phase acetonitrile-water (35:65, v / v), flow rate 0.4mL / min, column temperature 35°C, detection wavelength 238nm, see attached chromatogram figure 1 . Under this condition, the regression equation of 4-chloro-N-(2-mercaptoethyl) benzamide is y=2284.6x-1474.9(R 2 =0.9999), y is the peak area, x is the concentration (μM) of 4-chloro-N-(2-mercaptoethyl) benzamide, R 2 is the correlation coefficient, the linear range is 2.5-200μM.
Embodiment 3
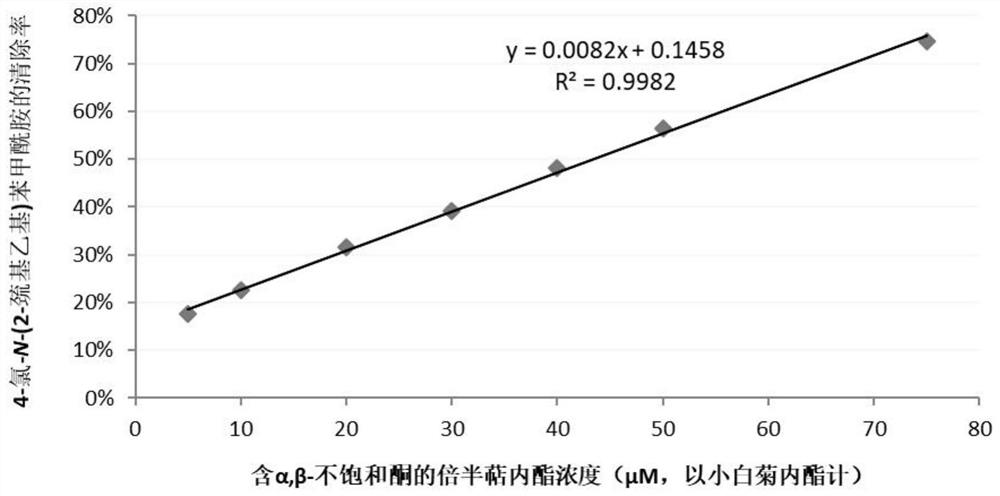
[0043] Establishment of Calibration Curve
[0044]Take an appropriate amount of parthenolide, weigh it accurately, dissolve it in a phosphate buffer (10mM, pH7.4)-methanol mixed solution (1:1, v / v), and prepare the concentration of 10, 20, 60, 80, 100, 150, 200μM Serial reference solution. Take an appropriate amount of 4-chloro-N-(2-mercaptoethyl)benzamide, accurately weigh it, dissolve it in a mixed solution of phosphate buffer (10mM, pH7.4)-methanol (1:1, v / v), and prepare into a solution with a concentration of 200 μM. Parthenolide solutions of different concentrations were mixed with an equal volume of 4-chloro-N-(2-mercaptoethyl)benzamide solution respectively, and reacted at 37°C for 120 minutes. Detect the remaining amount of 4-chloro-N-(2-mercaptoethyl)benzamide in the solution after 120 minutes of reaction, and calculate its clearance rate. With the concentration of parthenolide in the initial reaction solution as abscissa, with the clearance rate of 4-chloro-N-(2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com