Carbazochrome sodium sulfonate hydro-acupuncture injection as well as preparation method and application thereof
A technology of sodium carbosulfonate water and sodium carbosulfonate, which is applied in the field of sodium carbosulfonate water injection and its preparation, can solve the problems of being unable to tolerate terminal sterilization and low level of sterility assurance, and achieve improved resistance The effect of strong force, strong tolerance, and high content of sodium carbosulfonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
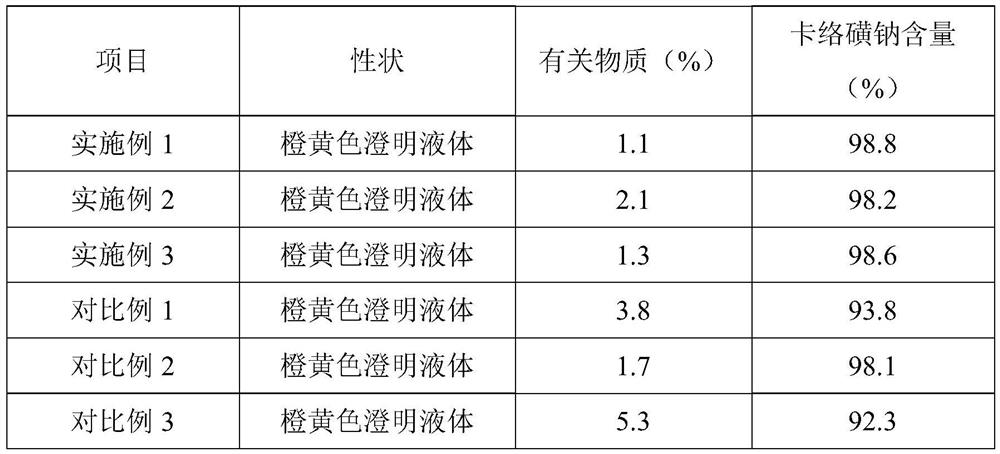
Embodiment 1
[0037] This embodiment provides an aqueous injection of sodium carbosulfonate, each milliliter of the injection includes: 5 mg of sodium carbosulfonate, 200 mg of ethanol, and 0.2 mg of disodium edetate.
[0038] The raw material composition of the above-mentioned sodium carbosulfonate aqueous injection includes: 5 g of sodium carbosulfonate, 200 g of ethanol, 0.2 g of disodium edetate, and the volume is adjusted to 1000 ml with water for injection; the preparation method includes the following steps:
[0039] (1) Take by weighing sodium carbosulfonate, ethanol, edetate disodium respectively according to the formula quantity, for subsequent use;
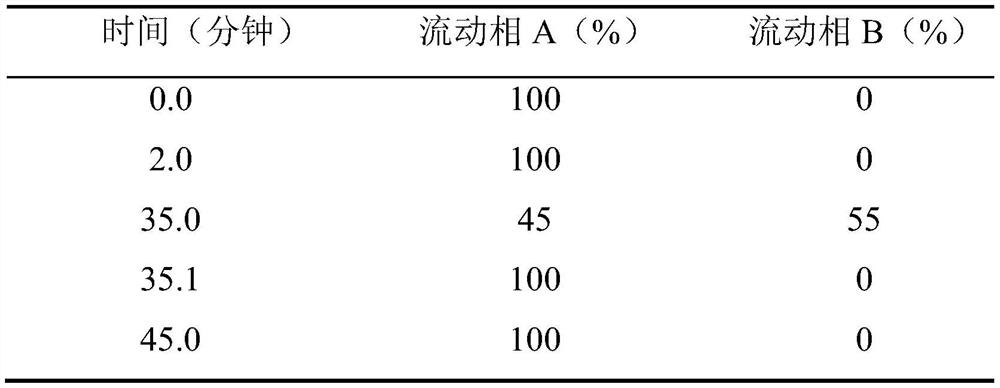
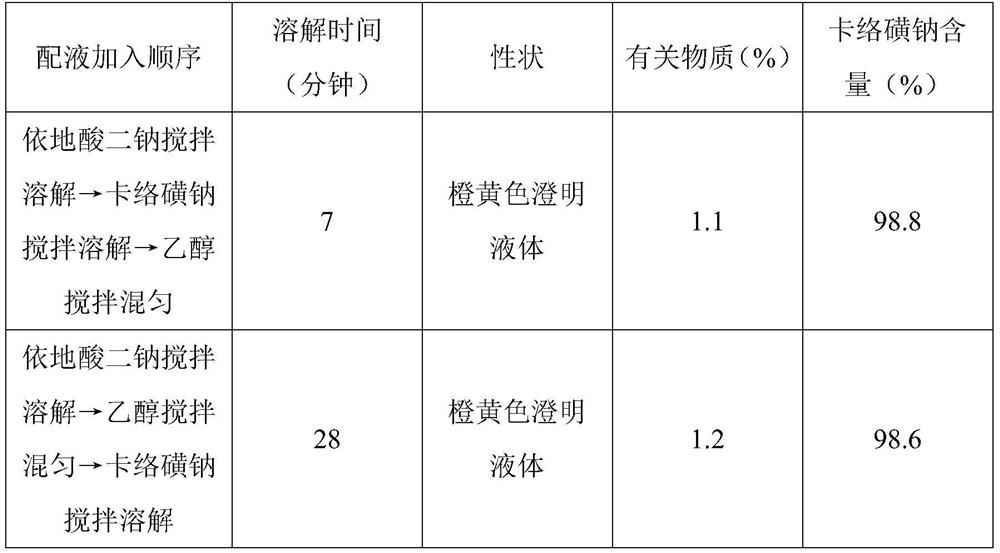
[0040] (2) Add water for injection of 70% of the prepared volume in the liquid mixing tank, pass into cooling water, make the temperature drop to 40°C, drop in disodium edetate, stir and dissolve, then drop in sodium carbosulfonate, stir and dissolve , put in ethanol, stir and mix evenly, use 0.01mol / L hydrochloric acid or sodium hyd...
Embodiment 2
[0043] This embodiment provides an aqueous injection of sodium carbene sulfonate, each milliliter of the injection includes: 5 mg of sodium carbene sulfonate, 200 mg of propylene glycol, and 0.2 mg of disodium edetate.
[0044] The raw material composition of the above-mentioned sodium carbosulfonate aqueous injection includes: 5 g of sodium carbosulfonate, 200 g of propylene glycol, 0.2 g of disodium edetate, and dilute to 1000 ml with water for injection; the preparation method includes the following steps:
[0045] (1) Take sodium carbosulfonate, propylene glycol, and disodium edetate by weighing respectively according to the formula quantity, and set aside;
[0046] (2) Add water for injection of 70% of the prepared volume in the liquid mixing tank, pass into cooling water, make the temperature drop to 50°C, drop in disodium edetate, stir and dissolve, then throw in sodium carbosulfonate, stir and dissolve , put in propylene glycol, stir and mix, use 0.01mol / L hydrochloric...
Embodiment 3
[0049] This embodiment provides an aqueous injection of carbosulfonate sodium, which includes: 5 mg of carbosulfonate sodium, 200 mg of ethanol, and 0.2 mg of calcium sodium edetate in each milliliter of the injection.
[0050]The raw material composition of the above-mentioned sodium carbosulfonate aqueous injection includes: 5 g of sodium carbosulfonate, 200 g of ethanol, 0.2 g of calcium sodium edetate, and dilute to 1000 ml with water for injection; the preparation method includes the following steps:
[0051] (1) Take by weighing sodium carbosulfonate, ethanol, calcium sodium edetate respectively according to the formula quantity, for subsequent use;
[0052] (2) Add water for injection of 70% of the prepared volume in the liquid mixing tank, pass into cooling water, make the temperature drop to 40°C, drop in edetate calcium sodium, after stirring and dissolving, drop into carbosulfonium sodium, after stirring and dissolving , put in ethanol, stir and mix evenly, use 0.01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com