A kind of rotaxane molecular machine based on crown ether and preparation method
A technology of molecular machines and rotaxanes, applied in chemical instruments and methods, instruments, organic chemistry, etc., can solve the problems of inaccuracy, limited effect, unintuitive detection methods, etc., and achieve the goal of reducing steric hindrance and sensitive detection methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] The present invention will be further described below with reference to the accompanying drawings and specific embodiments.
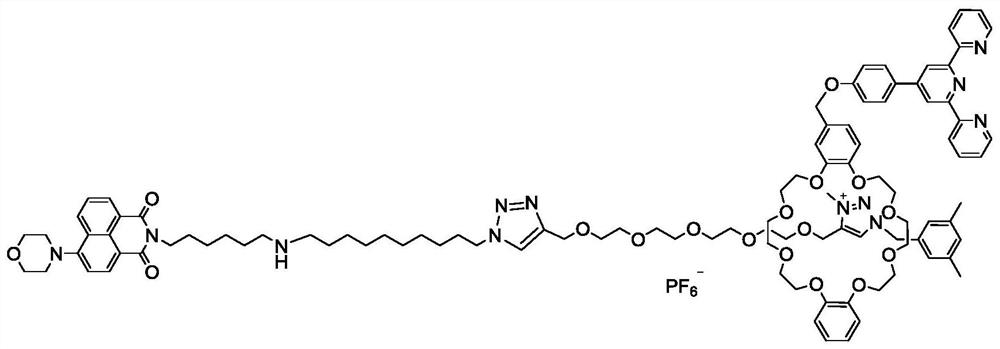
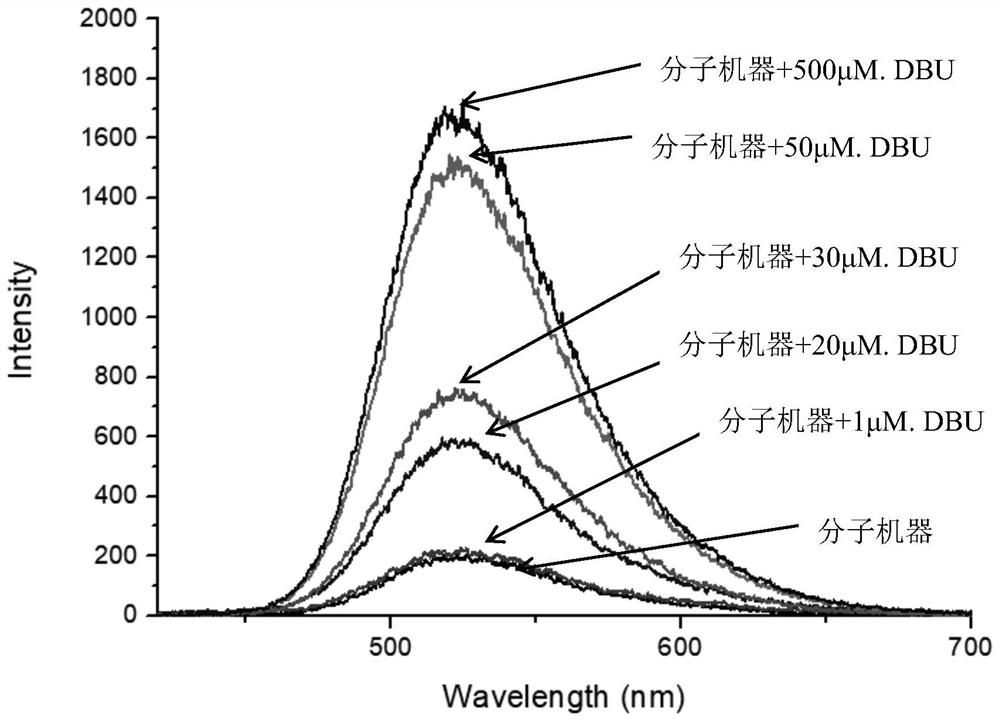
[0049] such as figure 1 The molecular machine shown is prepared from intermediate 1, intermediate 2, and intermediate 3, and the synthesis method is as follows:
[0050] Synthesis of 4-morpholine-1,8-dinaphthoic anhydride
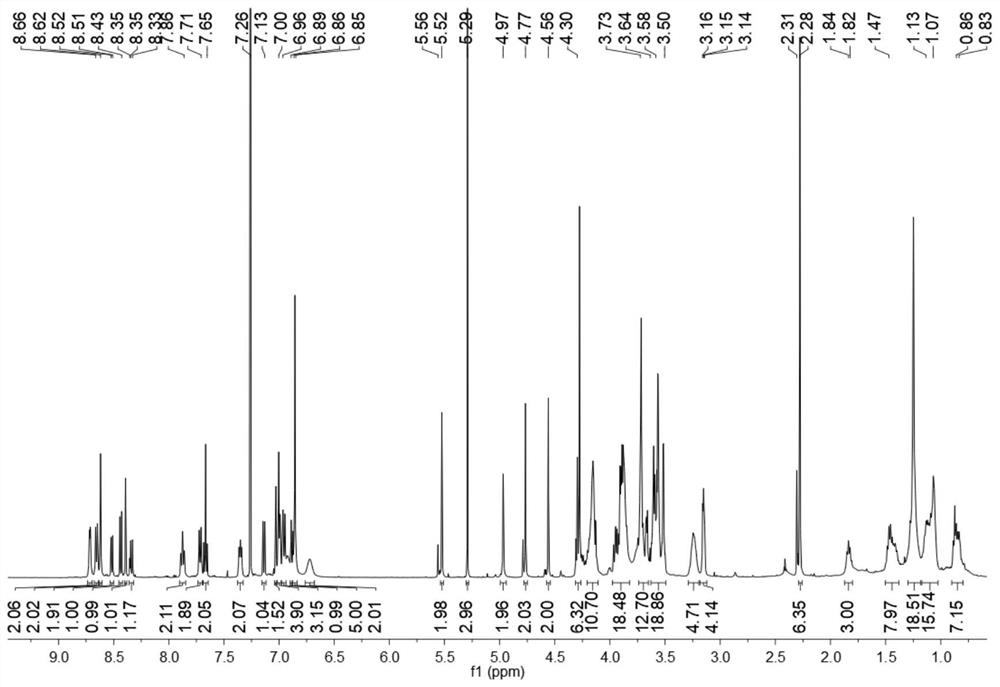
[0051] In a 100 mL round-bottomed flask, under nitrogen protection, 4-bromo-1,8-dinaphthoic anhydride (0.5 g, 1.80 mmol) was dissolved in 10 mL of ethylene glycol methyl ether solution, and 50 mg of cuprous iodide and morphine were added. Phosphate (0.47 g, 5.41 mmol), refluxed for 7 h, filtered and washed with ethanol several times to obtain compound 4 (0.29 g, 58%) as a yellow solid. TLC (developing solvent: petroleum ether: ethyl acetate / 1:1 / v:v) R f =0.39,1H NMR (400MHz, CDCl 3 )δ8.60(d, J=8.4Hz, 1H), 8.54(d, J=8.1Hz, 1H), 8.47(d, J=8.5Hz, 1H), 7.79-7.72(m, 1H), 7.27- 7.25 (d, J=8.3 Hz, 1H), 4.02 (d, J=4.7 Hz, 4H), 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com