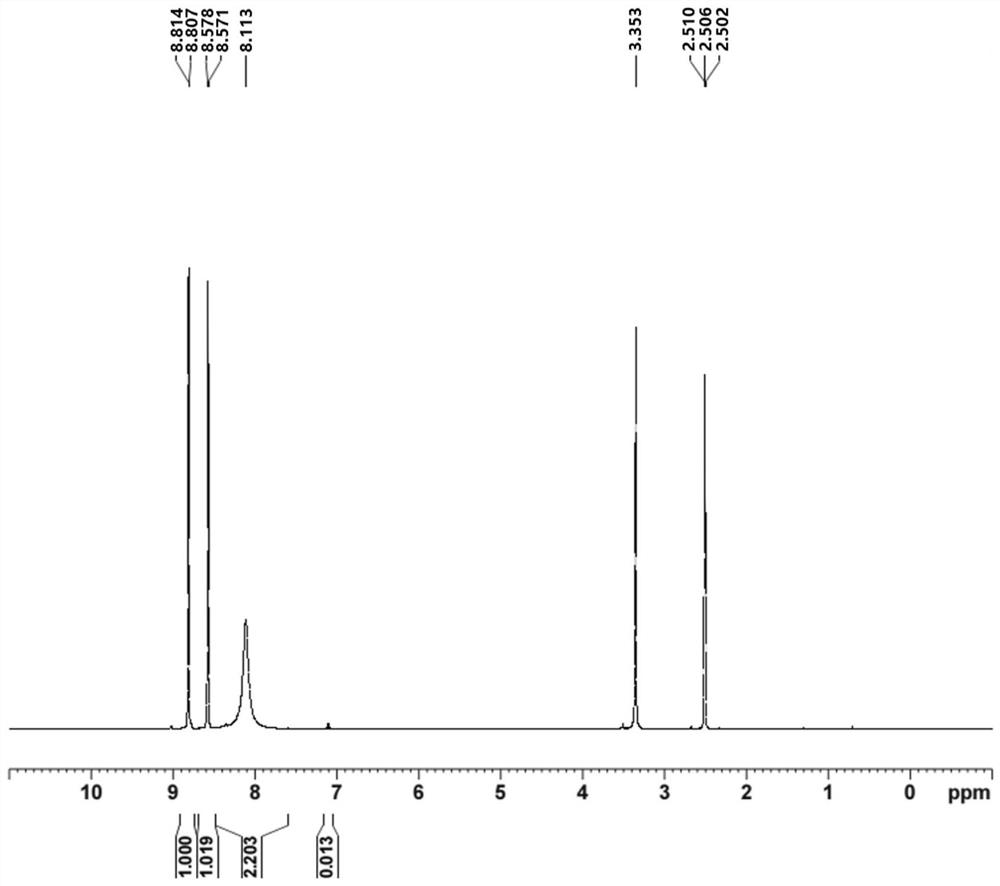
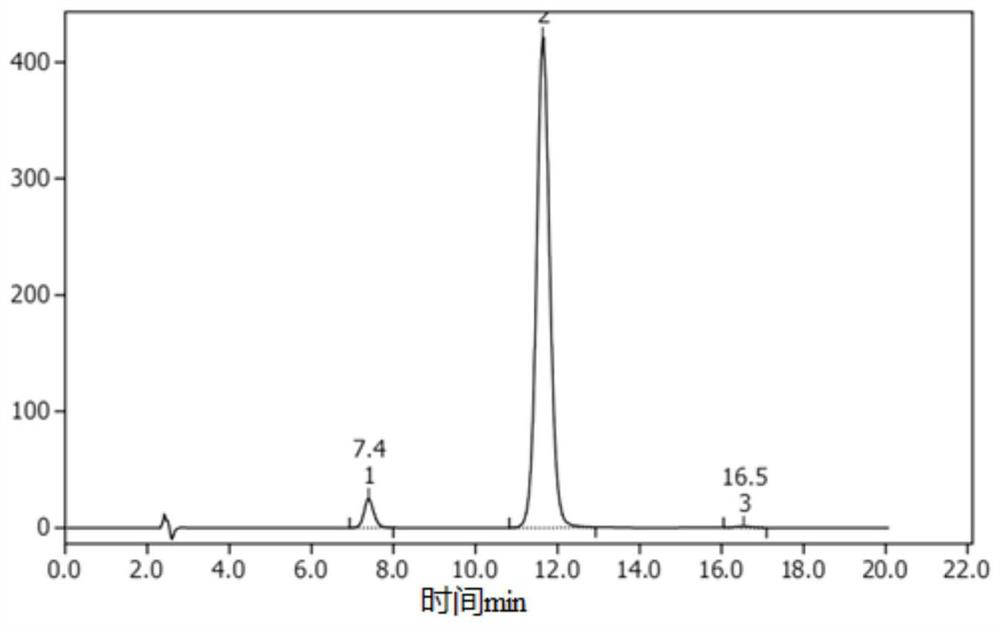
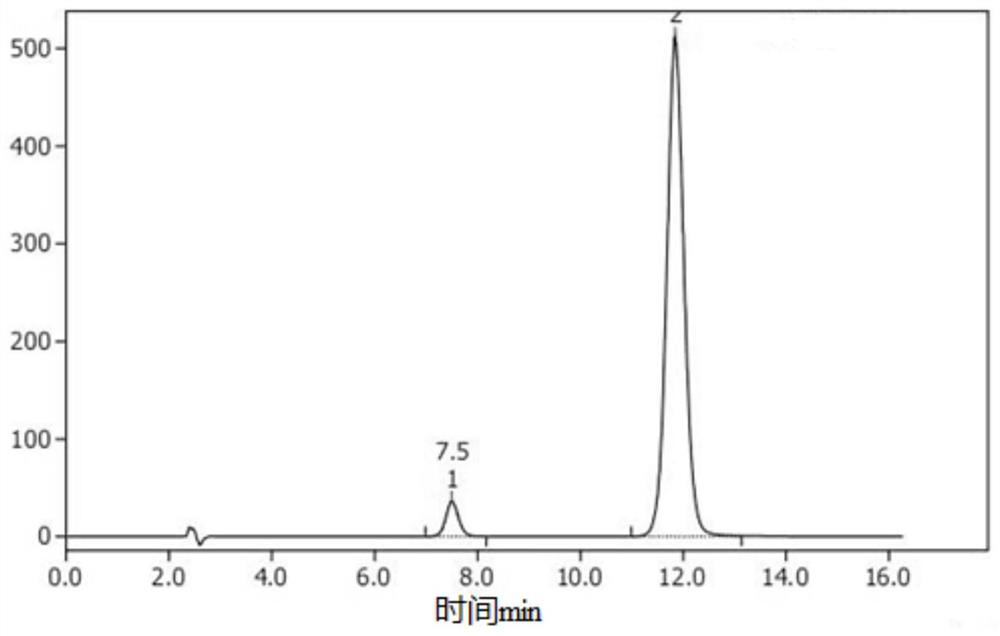
Synthesis process of 6-chloro-2, 4-dinitroaniline
A technology of dinitroaniline and synthesis process, which is applied in the preparation of amino compounds, purification/separation of amino compounds, preparation of organic compounds, etc., can solve the problems that sodium hypochlorite is not very stable, the cost of production raw materials is low, the productivity of equipment is not high, etc. Conducive to market competition, considerable economic benefits, significant effect of energy saving and emission reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of synthetic technique of 6-chloro-2,4-dinitroaniline, comprises the following steps:
[0037] 1) Add 150 mL of organic solvent ethyl acetate to the reaction kettle, then add 18 g of 2,4-dinitroaniline (the ratio is 8.3 L: 1 kg) to the ethyl acetate, mix well, and then add the concentration of 8 % hydrochloric acid solution 1g (the amount added is 5.56% of the mass of 2,4-dinitroaniline), stirred at 55°C for 1h to make a puree;
[0038] 2) Add 17.5g of dichlorohydantoin in batches (molar ratio: 0.9:1) to the original slurry under the condition of heat preservation at 55°C. The total time of addition is 1h, the amount of single addition is 0.875g, and the time interval is 3min. Then continue to react at 55°C for 1h;
[0039] 3) After the reaction is completed, concentrate to remove 50% of ethyl acetate, then cool to room temperature, filter, then wash the filter cake with purified water until the pH reaches 6-7, and finally dry to obtain the target product 6-chlo...
Embodiment 2
[0043]A kind of synthetic technique of 6-chloro-2,4-dinitroaniline, comprises the following steps:
[0044] 1) Add 600mL of organic solvent methanol into the reaction kettle, then add 40g of 2,4-dinitroaniline to the methanol, mix well, then add 6g of hydrochloric acid solution with a concentration of 5% to it, and stir at 45°C 1h, make puree;
[0045] 2) Add 23.6g of dichlorohydantoin in batches to the original slurry under the condition of heat preservation at 45°C, the total time of addition is 3h, the single addition amount is 1.18g, and the time interval is 9min, and then continue the reaction at 45°C 6h;
[0046] 3) After the reaction is completed, concentrate to remove 50% of methanol, then cool to room temperature, filter, then wash the filter cake with purified water until the pH reaches 6-7, and finally dry it to obtain the target product 6-chloro-2,4- Dinitroaniline 35.5g. The purity of the target product 6-chloro-2,4-dinitroaniline is ≥95.0%, and the yield is 74...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com