Method for preparing functionalized polysubstituted aromatic hydrocarbon through cascade reaction
A series reaction and multi-substitution technology is applied in the relay reaction between organic antimony compounds and polyhalogenated aromatic hydrocarbons to prepare functionalized multi-substituted aromatic hydrocarbons, and in the field of synthesizing functionalized multi-substituted aromatic hydrocarbons, which can solve the problems of unknown series reaction of organic antimony compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
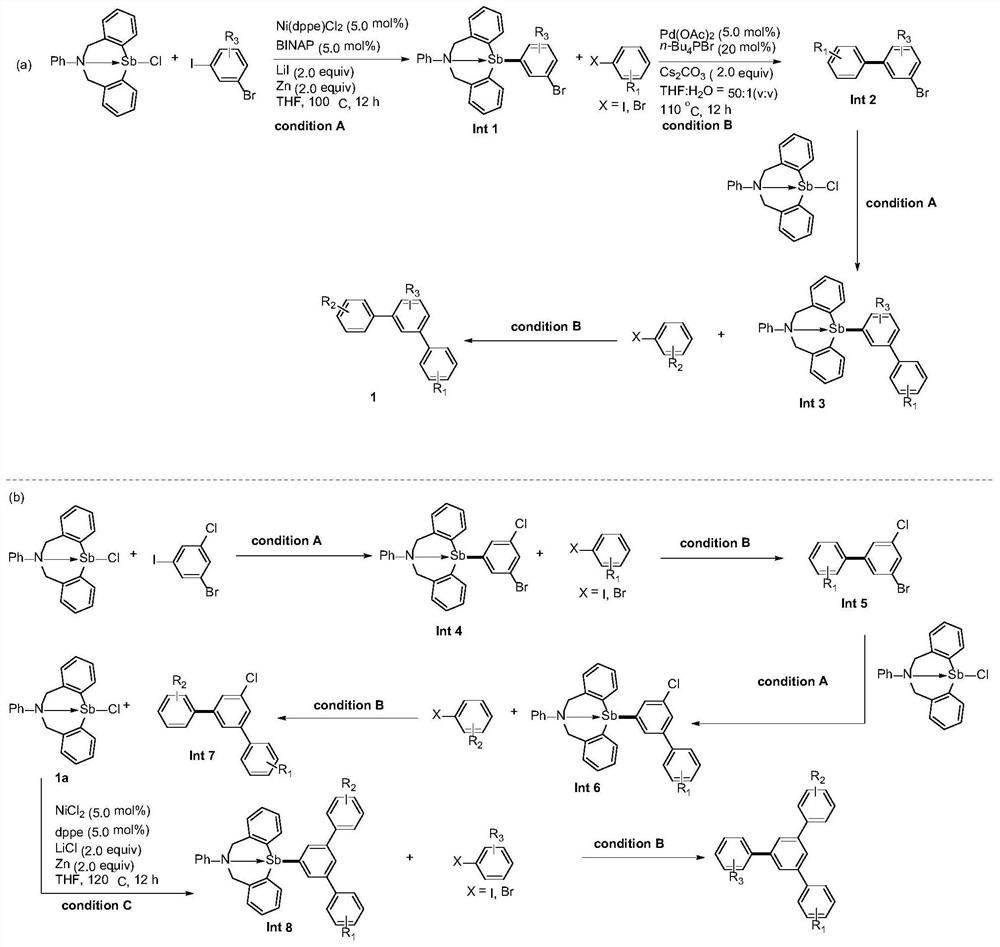
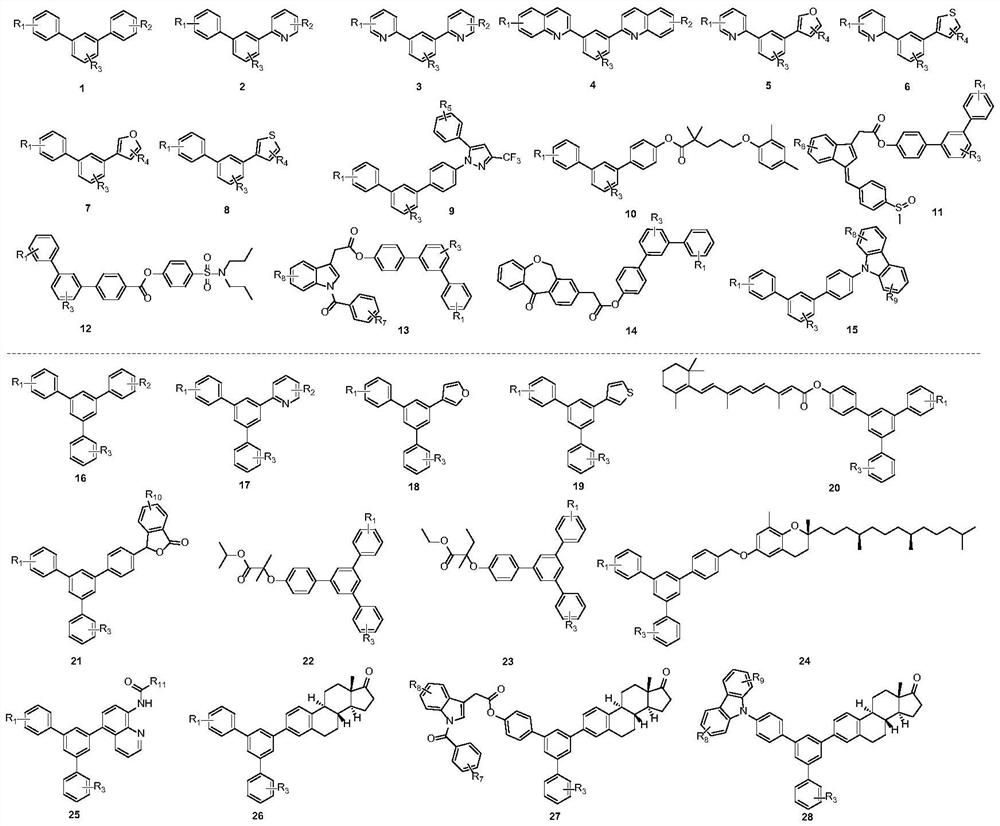
[0028] (1) Add organic antimony compound 55 (0.3 mmol), 1-bromo-3-iodo-5-toluene 29a (R 3 =5-Me) (0.3mmol), 1,2-bis(diphenylphosphine)ethane nickel chloride (0.015mmol), 1,1'-binaphthyl-2,2'-bisdiphenylphosphine (0.015 mmol), lithium iodide (0.6mmol), zinc powder (0.3mmol) and tetrahydrofuran (2.0mL), react at 100°C for 12h. After the reaction was completed, the solvent was removed under reduced pressure, and the pure compound was separated by column chromatography with a yield of 92%.
[0029] (2) the product (0.3mmol) obtained in the first step, 4-bromobenzonitrile 54a (R 2 =4-CN) (0.3mmol), palladium acetate (0.015mmol), n-butylphosphine bromide (0.06mmol), cesium carbonate (0.6mmol) and tetrahydrofuran (2.0mL) were added in the 25 ml reactor according to the proportion , stirred at 110°C for 12 hours. After the reaction was completed, the solvent was removed under reduced pressure, and the pure compound was obtained by column chromatography with a yield of 91%.
[0030...
preparation example 2
[0032] (1) Add organic antimony compound 55 (0.3 mmol), 1-bromo-3-iodo-5-ethylbenzene 29b (R 3 =5-Et) (0.3mmol), 1,2-bis(diphenylphosphine)ethane nickel chloride (0.015mmol), 1,1'-binaphthyl-2,2'-bisdiphenylphosphine (0.015 mmol), lithium iodide (0.6mmol), zinc powder (0.3mmol) and tetrahydrofuran (2.0mL), react at 100°C for 12h. After the reaction was completed, the solvent was removed under reduced pressure, and the pure compound was separated by column chromatography with a yield of 94%.
[0033] (2) the product (0.3mmol) obtained in the first step, 4-bromobenzonitrile 54a (R 2=4-CN) (0.3mmol), palladium acetate (0.015mmol), n-butylphosphine bromide (0.06mmol), cesium carbonate (0.6mmol) and tetrahydrofuran (2.0mL) were added in the 25 ml reactor according to the proportion , stirred at 110°C for 12 hours. After the reaction was completed, the solvent was removed under reduced pressure, and the pure compound was obtained by column chromatography with a yield of 92%.
[...
preparation example 3
[0036] (1) Add organic antimony compound 55 (0.3 mmol), 1-bromo-3-iodo-5-butylbenzene 29c (R 3 =5-Bu) (0.3mmol), 1,2-bis(diphenylphosphine)ethane nickel chloride (0.015mmol), 1,1'-binaphthyl-2,2'-bisdiphenylphosphine (0.015 mmol), lithium iodide (0.6mmol), zinc powder (0.3mmol) and tetrahydrofuran (2.0mL), react at 100°C for 12h. After the reaction was completed, the solvent was removed under reduced pressure, and the pure compound was obtained by column chromatography with a yield of 90%.
[0037] (2) the product (0.3mmol) obtained in the first step, 4-bromobenzonitrile 54a (R 2 =4-CN) (0.3mmol), palladium acetate (0.015mmol), n-butylphosphine bromide (0.06mmol), cesium carbonate (0.6mmol) and tetrahydrofuran (2.0mL) were added in the 25 ml reactor according to the proportion , stirred at 110°C for 12 hours. After the reaction was completed, the solvent was removed under reduced pressure, and the pure compound was obtained by column chromatography with a yield of 89%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com