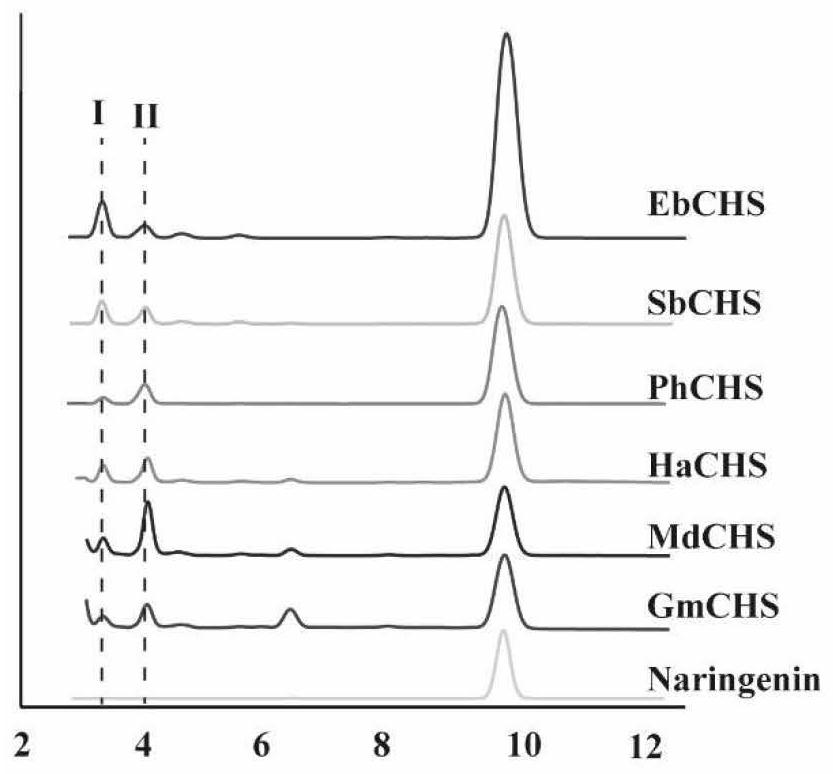
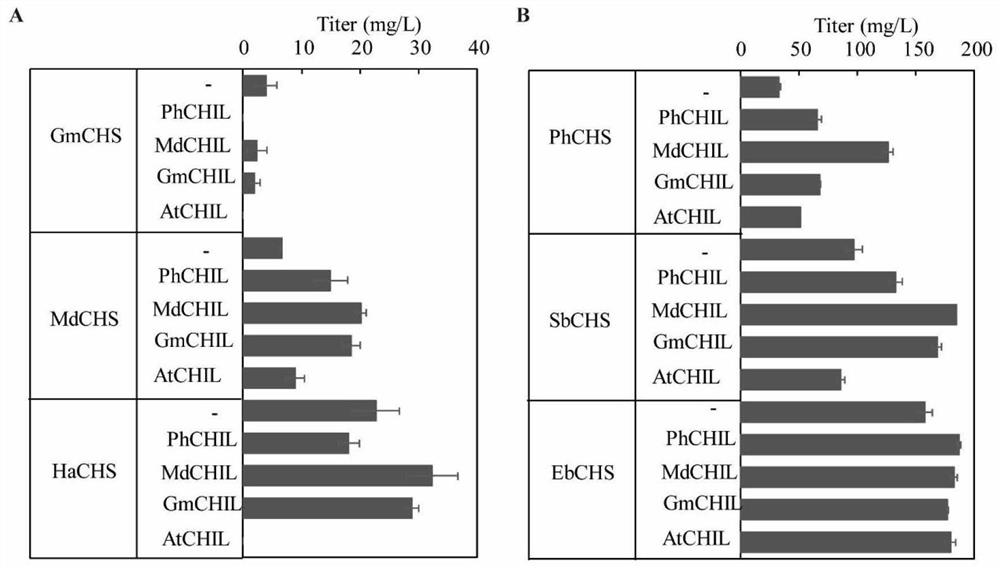
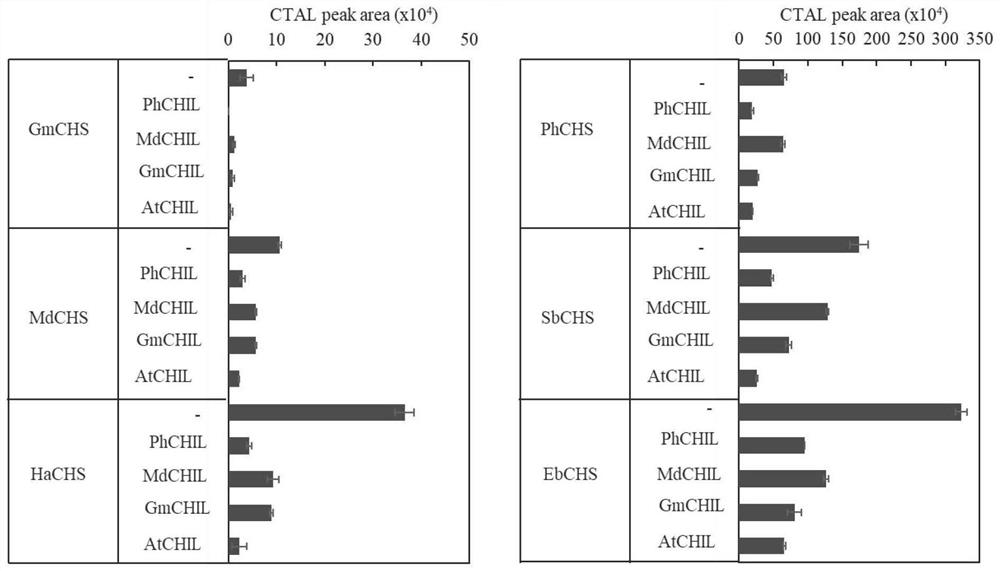
Enzyme composition and application thereof in naringenin biosynthesis
An enzyme composition, chalcone synthase technology, applied in the biological field, can solve the problem of enhancing plant anthocyanins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1 constructs the expression vector of CHSs
[0086] According to the amino acid sequence of the chalcone isomerase ErCHI (4D06_A) of Eubacterium ramulus, combined with the codon preference of Escherichia coli, and removing the commonly used enzyme cutting sites, the full-length Erchi gene was designed, and the Erchi The gene is connected between the BamHI / HindIII restriction sites of the pETDuet-1 plasmid, and fully artificially synthesized to obtain the vector plasmid pNR1.
[0087] According to the amino acid sequence of soybean (Glycine max) chalcone synthase GmCHS (NP_001340309.1), combined with the codon preference of Escherichia coli, and removing the commonly used enzyme cutting sites, the full-length Gmchs gene was designed, and the Gmchs gene was connected Between the NdeI / XhoI restriction sites of the pNR1 plasmid, it was completely artificially synthesized to obtain the vector plasmid pNR2.
[0088] According to the amino acid sequence of the chal...
Embodiment 2
[0093] Embodiment 2 constructs the expression vector of CHILs
[0094] According to the amino acid sequence of the 4-coumaryl-CoA ligase At4CL (U18675) of Arabidopsis thaliana, combined with the codon preference of Escherichia coli, and removing the commonly used enzyme cutting sites, the full-length At4cl gene was designed, The At4cl gene was connected between the NdeI / XhoI restriction sites of the pCDFDuet-1 plasmid and fully artificially synthesized to obtain the vector plasmid pNR8.
[0095] According to the amino acid sequence of petunia's chalcone isomerase-like protein PhCHIL (BAJ10400.1), combined with the codon preference of Escherichia coli, and removing the commonly used enzyme cutting sites, the full-length Phchil gene was designed, and the Phchil gene was It was connected between the NcoI / BamHI restriction sites of the pNR8 plasmid and fully artificially synthesized to obtain the vector plasmid pNR9.
[0096] According to the amino acid sequence of the apple chal...
Embodiment 3
[0099] Example 3 Construction of recombinant strains expressing CHILs and CHSs
[0100] Plasmids pNR2-pNR7 and pNR8 were transformed into E. coli E.coli BL21 (DE3) competent at the same time, and the competent cells were cultured overnight on LB solid medium containing streptavidin and ampicillin, and positive clones were screened to construct a control Strains BNR1-BNR6 were kept for future use.
[0101] Plasmids pNR2-pNR7 and pNR9-pNR12 were transformed into E. coli E.coli BL21 (DE3) competent at the same time, respectively, and the competent cells were cultured overnight on LB solid medium containing streptavidin and ampicillin, and positive clones were screened. The fermentation strain BNR7-BNR30 was constructed and stored for future use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com