EMA-ddPCR primer and probe for detecting infectious ASFV and application
A probe and positive technology, applied in the direction of DNA / RNA fragments, recombinant DNA technology, microbial determination / inspection, etc., can solve the problem of reducing PCR amplification efficiency and sensitivity, GC content, different base preference conformational characteristics, EMA binding Efficiency is not the same problem, to achieve the effect of shortening the test cycle, good application prospects, and reducing false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
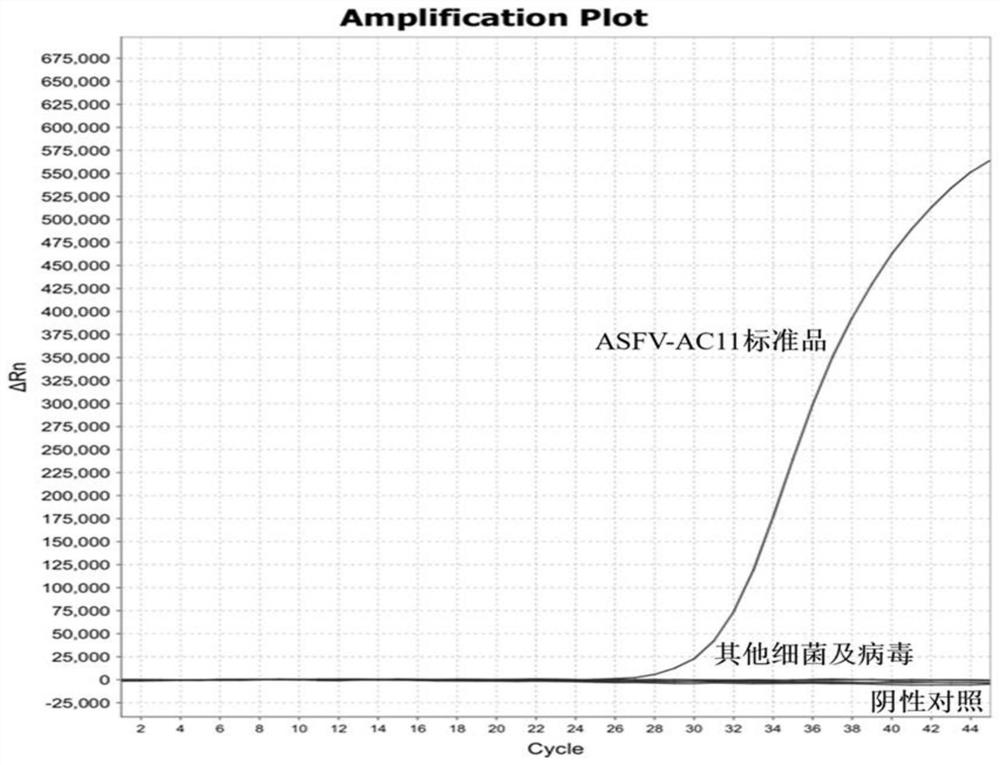
[0019] Detecting infectious ASFV amplified fragment screening, lead optimization and specificity of test probes:
[0020] 1.1 Design of ASFV infection with the reference primers and probes
[0021] The applicant ASFV genome, the 27 selected target sequence to design primers and probes, designed primer probe combination designated ASFV-AC1 ~ ASFV-AC27; primer and probe sequences were SEQ ID NO.1 ~ SEQ IDNO.81 shown; e.g. ASFV-AC1 the primers shown in SEQ ID NO.1 ~ SEQ ID NO.2, SEQ ID NO.3 probe is shown; the remaining composition and so on.
[0022] 19 are simultaneously selected combination of the detection probe ASFV conventional primers, designated ASFV-ZL1 ~ ASFV-ZL15, wherein:
[0023] ASFV-ZL1 primer probe set derived from the primer and probes of claim CN2020100456541 requirements;
[0024] ASFV-ZL2 primer probe set of primers and probes derived from the P72 gene of claim 4 CN111172321A the requirements;
[0025] ASFV-ZL3 primer probe set of primers and probes derived from g...
Embodiment 2
[0076] EMA ASFV optimization method for detecting infectious: Example 2
[0077] 2.1EMA concentration optimization
[0078] An equal volume were pipetted complete inactivation of the test sample specimen, with different concentrations EMA (final concentration 0,0.25,0.5,1,1.5,2,2.5,3μg / mL) were incubated in the dark at 4 ℃ swirl 30min, the end of incubation after using the PMA-Lite TM Solutions of the sample analyzer of the LED light photolysis 20min. Tiangen using automated nucleic acid extractor to extract nucleic acid, and thus best performed using qRT-PCR reaction system and conditions obtained in Example 1 as a template. Screening can detect the lowest concentration E MA infectious ASFV purposes.
[0079] Table 4 EMA concentration optimization
[0080] EMA final concentration (μg / ml) CT value average ± standard deviation △ CT 0 33.523±0.016 0 0.25 35.923±0.128 2.4 0.5 36.237±0.155 2.714 1 36.923±0.052 3.4 1.5 38.245±0.128 4.722 ...
Embodiment 3
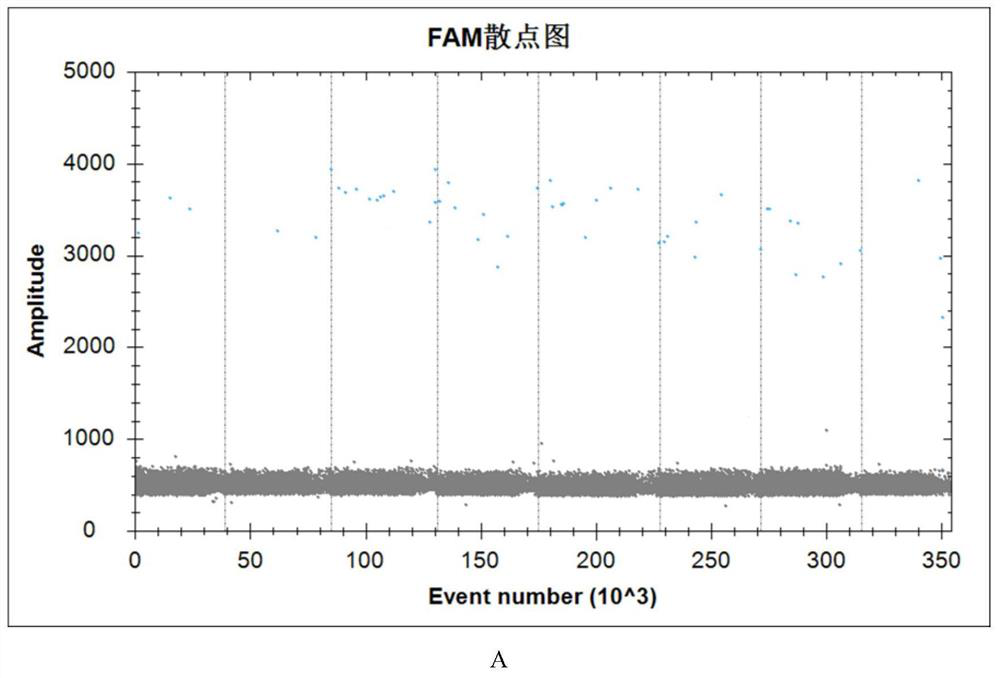
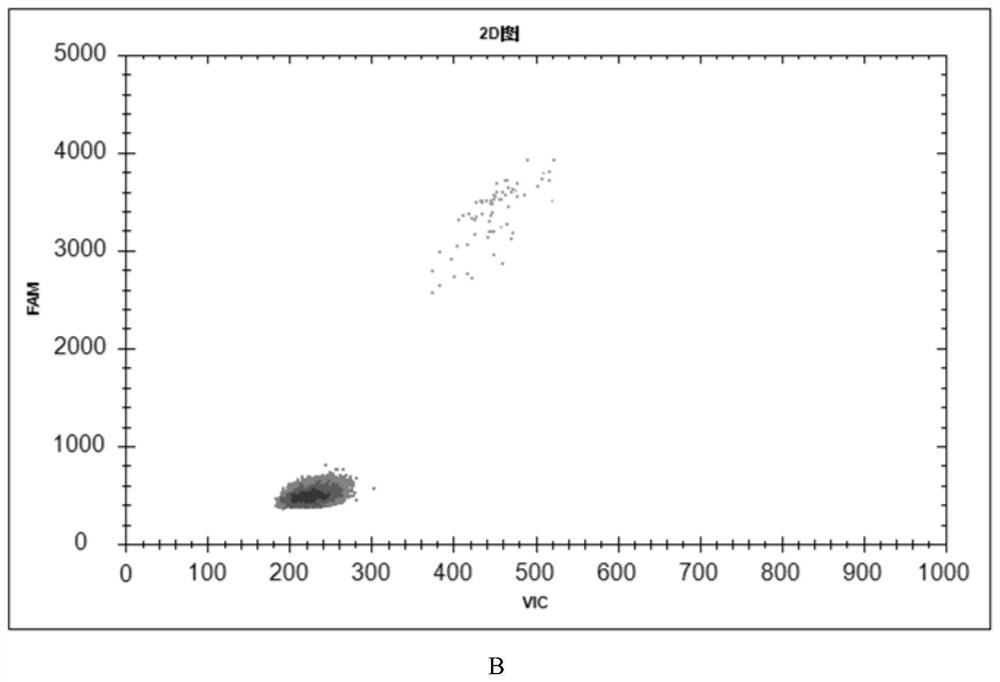
[0090] Example 3: Application of microfluidic chip-based detection kit infectious ASFVddPCR
[0091] This reference to the embodiments of rural China Ministry of Agriculture issued the "outbreak of African swine fever contingency embodiment (second edition 2020)" and the Chinese Academy of Agricultural Sciences released "African swine fever cleaning and disinfection Techniques (Second Edition)" mentioned ASFV standard sterilization, disinfection active substance content, disinfectant concentration and disinfectant action time analog aldehyde (formaldehyde), an alcohol disinfectant (75% ethanol), chlorine-containing disinfectant (84 disinfectant), quaternary ammonium salts disinfectant (benzalkonium chloride), a peroxide-based disinfectant (hydrogen peroxide), potassium hydrogen-based (oxone) inactivated sample (erythrocyte assay was negative after verification disinfectants as inactivated complete group), taking additional viral ASFV erythrocyte adsorption test was positive for th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com