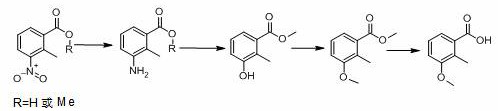
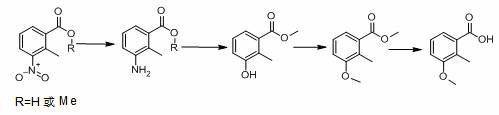
Synthesis process of 2-methyl-3-methoxybenzoic acid
A technology of methoxybenzoic acid and toluic acid, which is applied in the field of synthesis of organic pesticide intermediates, can solve problems such as difficult to meet the production demand of the pesticide methoxyfenozide, unstable product quality, corrosion of equipment and pipelines, and achieve Product quality is stable and controllable, meets quality requirements, and reduces usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Reductive hydrogenation
[0043] 3-nitro-2-methylbenzoic acid (500 g), platinum carbon (platinum content 2%, 50 g), methanol (2000 g), add to the autoclave, replace with nitrogen, and pass hydrogen to 1.0-1.3 MPa , raise the temperature to 70-80°C, control the temperature and control the reaction pressure until the reaction is complete (raw material <0.5%), continue to stir for 60 min, filter while hot, collect the catalyst for mechanical use, and directly carry out diazotization hydrolysis of the mother liquor.
[0044] (2) Diazotization hydrolysis
[0045] The temperature of the reduction mother liquor obtained in step (1) is lowered to 0-5°C, and 900 g of nitrosyl sulfuric acid (weight percentage: 40%, 1.02 eq.) is added dropwise under temperature control. 30min, after stirring for 1 hour, heat up to 64-66°C and reflux for 4-8 hours until the reaction is complete (HPLC: 3-hydroxy-2-methylbenzoic acid+3-methoxy-2-methylbenzoic acid<1 %).
[0046] The temperatur...
Embodiment 2
[0054] (1) Reductive hydrogenation
[0055] Add 3-nitro-2-methylbenzoic acid (450 g), platinum carbon (platinum content 2%, 9.0 g), methanol (1350 g), into the autoclave, nitrogen replacement, hydrogen pressure to 0.9-1.1 MPa , raise the temperature to 60-70°C, control the temperature and control the reaction pressure until the reaction is complete (raw material <0.5%), continue to stir for 30 min, filter while hot, divide the mother liquor into three parts while hot, and directly carry out diazotization hydrolysis.
[0056] (2) Diazotization hydrolysis
[0057] Add 92.2 g (1.2 eq.) of sulfuric acid to the reduction mother liquor (1 / 3 amount) obtained in step (1), cool down to 5-10°C, and add 126.6 g of sodium nitrite solution dropwise under temperature control (sodium nitrite: 63.6 g, 1.2 eq. .; Water: 63 g), after adding, slowly raise the temperature to 50-60°C, control the heating time for not less than 30min, stir for 1 hour, then raise the temperature to 64-66°C and refl...
Embodiment 3
[0063] diazotization hydrolysis
[0064] Add 150.6 g (2.0 eq.) of sulfuric acid to the reduction mother liquor obtained in step (1) of Example 2, add 150.6 g (2.0 eq.) of sulfuric acid, lower the temperature to 20-30°C, and add 155.7 g of sodium nitrite solution dropwise under temperature control (sodium nitrite: 55.7 g, 1.05 eq.; water: 100 g), after the addition, slowly raise the temperature to 50-60°C, stir for 1 hour, then raise the temperature to 64-66°C and reflux for 8-16 hours until the reaction is complete (HPLC: 3-hydroxy -2-methylbenzoic acid + 3-methoxy-2-methylbenzoic acid <1%).
[0065] Raise the temperature to 85-100°C, recover about 360 g of methanol by atmospheric distillation, add 400 g of water, let stand to separate layers, and separate 105 g of oil layer.
[0066] The aqueous layer was extracted with MIBK 100ml, the organic layer was separated and concentrated, and then combined with the oil layer to obtain 138 g of crude 3-hydroxy-2-methylbenzoate (HPLC:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com