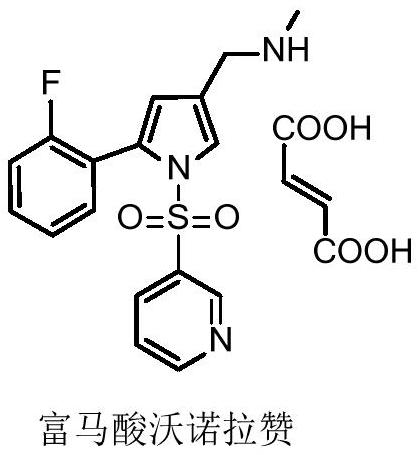
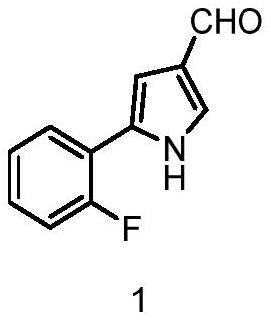
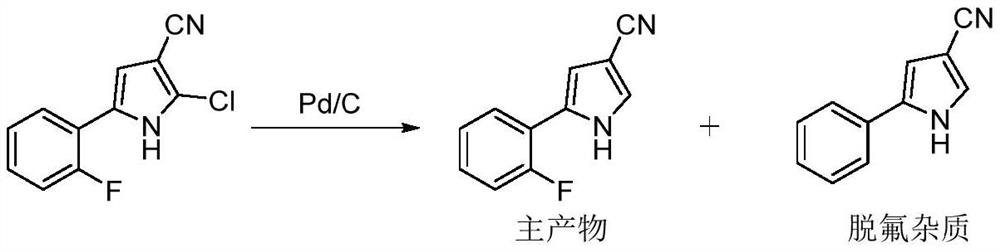
Preparation method of 5-(2-fluorophenyl)-1H-pyrrole-3-formaldehyde
A technology of fluorophenyl and pyrrole, which is applied in the field of preparation of vonoprazan fumaric acid intermediates, can solve the problems of cumbersome post-processing steps, affecting the quality of intermediates, and declining product yields, and achieves simple post-processing steps, The effect of improving product quality and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 106.8 kg of tetrahydrofuran and 11.00 kg of 2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile into the container, then add 3.00 kg of acetic acid, 4.09 kg of anhydrous sodium acetate, and a mass fraction of 3 % palladium carbon 1.10kg, and rinse the inlet with tetrahydrofuran 5.00kg. Introduce hydrogen to maintain the pressure in the kettle at 0.4±0.1MPa, control the temperature at 30±10°C and stir for 20 hours. After the reaction, the raw materials are completely reacted by HPLC monitoring. The monitoring results show that the HPLC purity of the reaction liquid is 90.03%, and the defluorinated impurity content is 0.03%. The hydrogen in the kettle was evacuated, and then the hydrogen in the kettle was completely replaced with nitrogen, and then the palladium carbon was filtered out. After the filtrate was concentrated to dryness under reduced pressure, absolute ethanol was added. After the temperature was raised to complete dissolution, purified water was added ...
Embodiment 2
[0046] Add 26.64 kg of tetrahydrofuran and 3.00 kg of 2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile into the container, then add 0.82 kg of acetic acid, 1.10 kg of anhydrous sodium acetate, and a mass fraction of 3 0.30kg of palladium carbon, feed hydrogen to keep the pressure in the kettle at 0.4±0.1MPa, control the temperature at 30±10°C and stir the reaction for 20 hours. The HPLC monitoring of the raw material reaction is complete. The monitoring results show that the HPLC purity of the reaction liquid is 89.73%, and the defluorinated impurities Content 0.04%. The hydrogen in the kettle was evacuated, and then the hydrogen in the kettle was completely replaced with nitrogen, and then the palladium carbon was filtered out. After the filtrate was concentrated to dryness under reduced pressure, absolute ethanol was added, and after the temperature was raised to complete dissolution, purified water was added dropwise to crystallize. After the dropwise addition was comp...
Embodiment 3
[0048] Add 20ml of tetrahydrofuran and 100.0g of 2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile into the container, then add 27.21g of glacial acetic acid, 37.18g of sodium acetate and 3% of Palladium carbon 10.0g. In a hydrogen atmosphere, after stirring and reacting at a temperature of 30±10° C. for 20 hours, the raw materials were completely reacted by HPLC monitoring. The monitoring results showed that the HPLC purity of the reaction liquid was 91.96%, and the content of defluorinated impurities was 0.04%. After the reaction is over, use diatomaceous earth to filter the palladium carbon, concentrate the filtrate to dryness under reduced pressure, add absolute ethanol, heat up to complete dissolution, add purified water dropwise to crystallize, after the dropwise addition is completed, keep warm for 1 hour after crystallization, filter, The resulting filter cake was dried to obtain the intermediate 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile, with an HPLC purity of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com