Pyridine amino hafnium compound as well as preparation method and application thereof
A technology for pyridylamino and hafnium compounds is applied in the field of pyridineamine-based hafnium compounds and their preparation, which can solve the problems of narrow application range of comonomers, and achieve the effects of simple and efficient ligand synthesis, improved solubility, and simple introduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
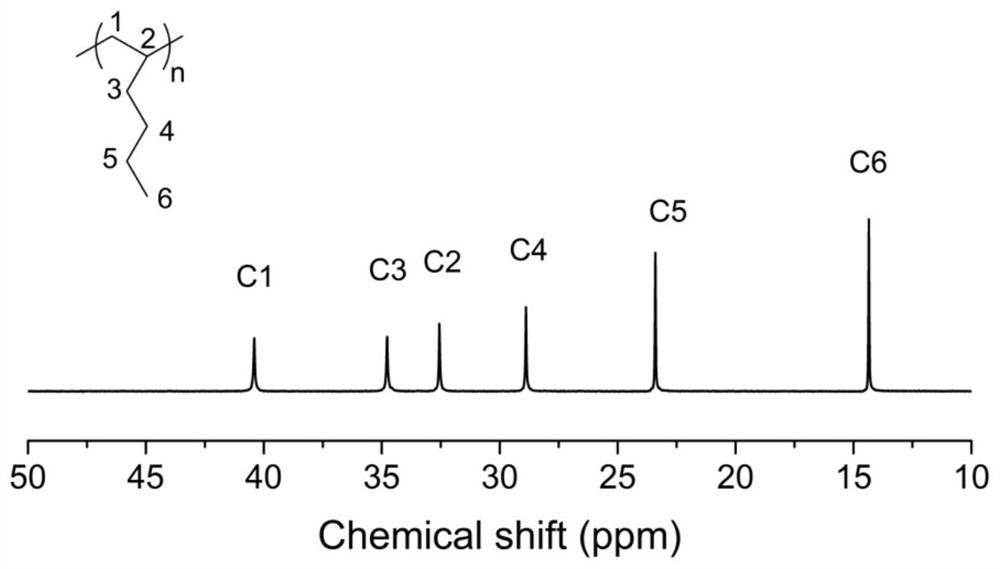
Examples
Embodiment 1
[0055] Synthesis of Pyridine Aldehyde Compound A1
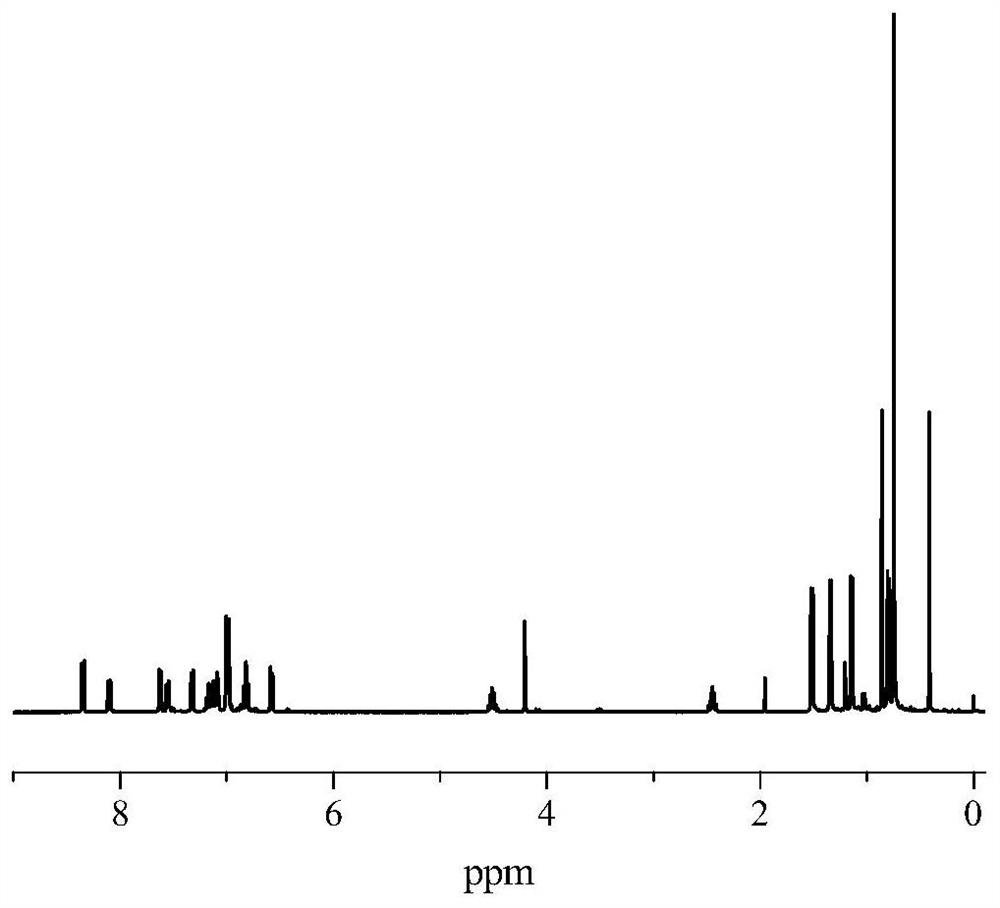
[0056] Under nitrogen atmosphere, add 1.86g (10mmol) of 6-bromopyridine-2 formaldehyde, 1.72g (10mmol) of naphthalene boronic acid, 15mg of tetrakis(triphenylphosphine)palladium, and 6g of potassium carbonate to the branch bottle successively, and pour into 30mL of ethanol , 20mL toluene, 10mL water, reflux overnight. Extract by liquid separation, wash with water, and dry to obtain 2.17 g of pyridine aldehyde compound A, with a yield of 93%. The H NMR spectrum is as follows: 1 H NMR (400MHz, CDCl 3 ):δ10.19(s,1H,CHO),8.00-8.07(m,3H,Py-H),7.92-7.99(m,2H,Ar-H),7.79-7.84(dd,1H,Ar-H ), 7.47-7.68 (m, 4H, Ar-H), 7.18-7.13 (m, 2H, ArH).
Embodiment 2
[0058] Synthesis of Pyridine Aldehyde Compound A2
[0059] According to the method in Example 1, naphthaleneboronic acid was replaced by phenylboronic acid.
[0060] The H NMR spectrum is as follows: 1 H NMR (400MHz, CDCl 3 ):δ10.17(s,1H,CHO),8.00-8.07(m,3H,Py-H),7.92-7.99(m,2H,Ar-H),7.79-7.84(dd,1H,Ar-H ), 7.47-7.13 (m, 2H, ArH).
Embodiment 3
[0062] Synthesis of Pyridinimine Compound B1
[0063] Under nitrogen atmosphere, 2.33g (10mmol) of pyridine aldehyde compound A1, 1.86g (10.5mmol) of 2,6-diisopropylaniline and 10mg of p-toluenesulfonic acid were dissolved in 50mL of toluene, and the water was refluxed for 48h. The solvent was spin-dried and dried to obtain the pyridine imine compound B with a yield of 94%. The H NMR spectrum is as follows: 1 H NMR (CDCl 3 ,400MHz):δ8.17(d,3H,Py-H),7.94(d,2H,Py-H),7.34-7.64(m,6H,Ar-H),6.76-6.94(m,4H,Ar -H),3.39(s,2H,CH(CH 3 ) 2 ),1.26(s,9H,C(CH 3 ) 3 ),1.15(d,6H,CH(CH 3 ) 2 ),0.86(d,6H,CH(CH 3 ) 2 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
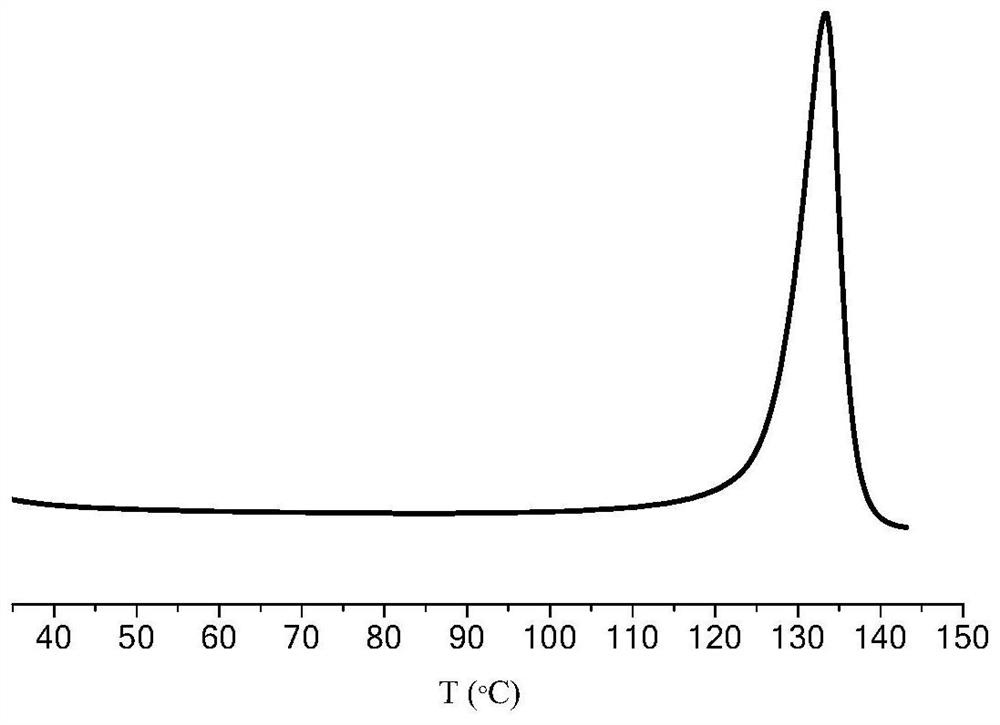
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com