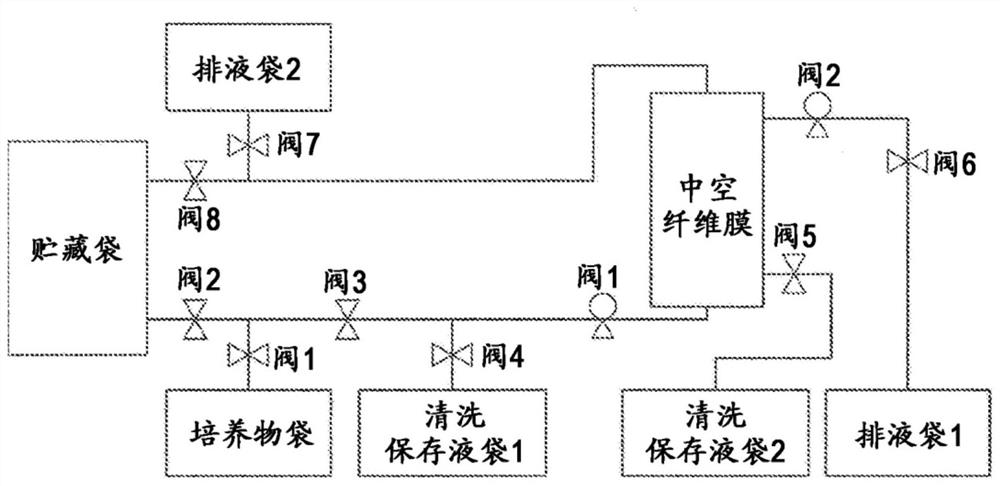
Storage liquid for mammalian cells
A mammalian and preservation solution technology, applied in the field of mammalian cell preservation solution, can solve the problem of PD-1 expression reduction and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] [Production of platelets derived from iPS cells]
[0079] Platelets derived from iPS cells were prepared according to the method described in PCT / JP2018 / 034667. The specific procedure is shown in (1-1) to (1-12) below.
[0080] (1-1) Preparation of hematopoietic precursor cells from iPS cells
[0081]According to the method of Takayama et al. (J.Exp.Med., 2010, vol.13, 2817-2830), from human iPS cells (TKDN SeV2 and NIH5: iPS derived from human fetal skin fibroblasts established using Sendai virus) cells) to differentiate into blood cells. Specifically, human ES / iPS cell colonies were co-cultured with C3H10T1 / 2 feeder cells in the presence of 20 ng / mL VEGF (manufactured by R&D SYSTEMS) for 14 days to produce hematopoietic progenitor cells (HPCs). . The above culture was carried out at 37°C, 20% O 2 , 5%CO 2 implemented under the conditions.
[0082] (1-2) Gene introduction system
[0083] The gene transfer system utilizes a lentiviral vector system. The lentivi...
Embodiment 2
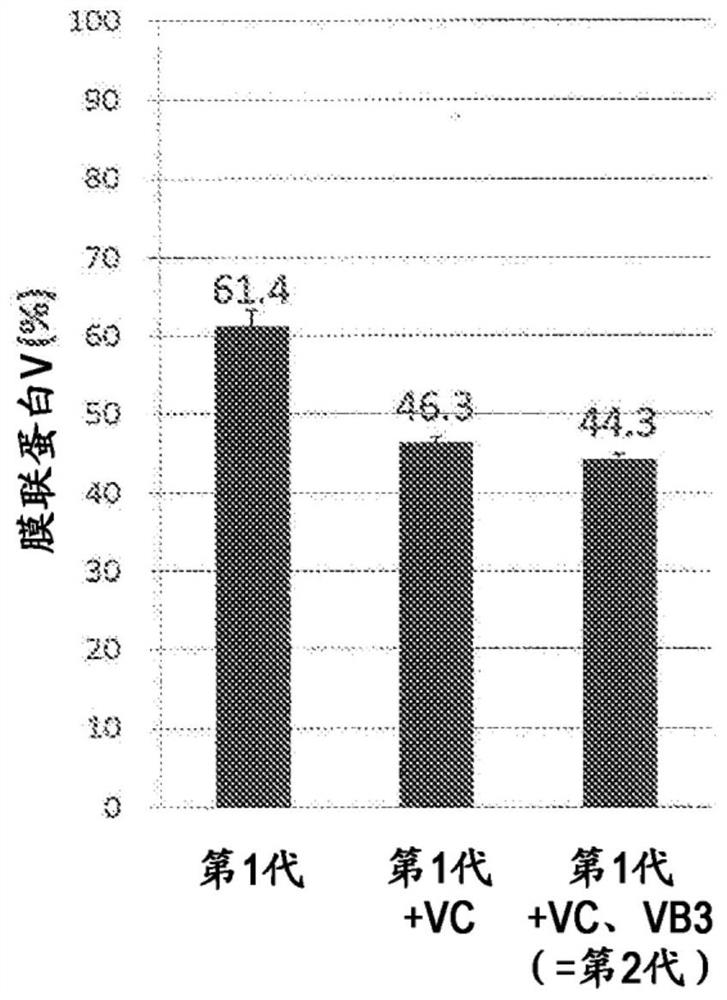
[0142] [Add vitamin C to the platelet storage solution]
[0143] (2-1) Preparation of platelet preservation solution
[0144] Bicarbonate Ringer's solution (BICANATE infusion solution; manufactured by Otsuka Pharmaceutical Factory, Inc.) (sodium chloride 5.84 g / L, potassium chloride 0.30 g / L, calcium chloride hydrate 0.22 g / L, magnesium chloride 0.20g / L, sodium bicarbonate 2.35g / L, and sodium citrate hydrate 0.20g / L), add human serum albumin preparation (HSA; CSL Behring company manufacture), and blood preservation solution (ACD-A liquid; Terumo Co., Ltd.) (2.20 W / V% of sodium citrate hydrate, 0.80 W / V% of citric acid hydrate, and 2.20 W / V% of glucose). In this specification, this solution is also referred to as "first-generation preservation solution". Separately, a solution obtained by adding VC preparation (injection preparation containing additives; manufactured by Sawai Pharmaceutical Co., Ltd.) to the first-generation preservation solution was prepared. In this specif...
Embodiment 3
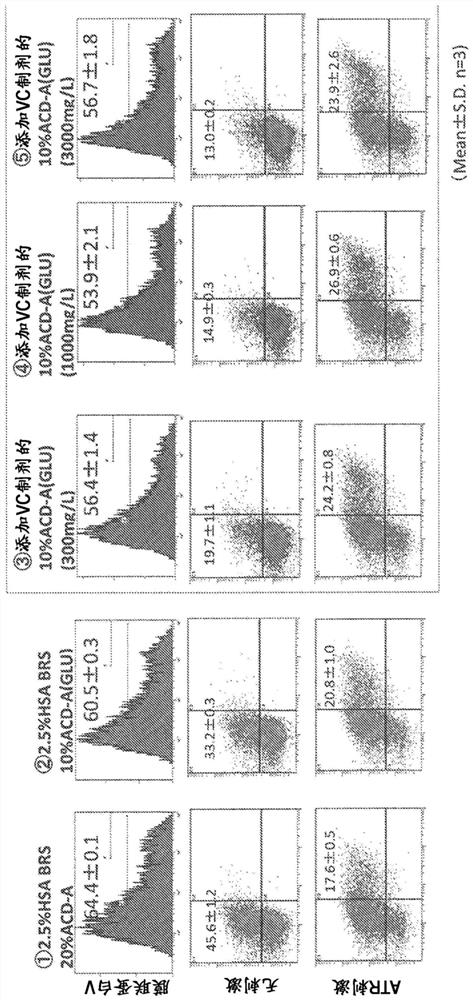
[0160] [Add VC and VB3 to platelet preservation solution]
[0161] (3-1) Preparation of platelet preservation solution
[0162] A solution was prepared by adding nicotinic acid (niacin injection preparation; manufactured by TOA EIYO Co., Ltd.) to the VC-added preservation solution. It should be noted that, as mentioned above, in the specification of this application, "VB3" means nicotinic acid and / or nicotinamide. Niacin is used as VB3 in Examples 3-5 and 8-13, and nicotinic acid is used as VB3 in Example 6. acid or nicotinamide as VB3. In addition, in this specification, a solution obtained by adding VB3 (nicotinic acid or nicotinamide) to a VC-added storage solution is also referred to as a "VC / VB3-added storage solution" or a "second-generation storage solution". The final concentrations of the above-mentioned additives in each preservation solution are shown in Table 3 below. In addition, any preservation solution was adjusted to pH 7.3±0.1 using 1M NaOH, and incubated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com