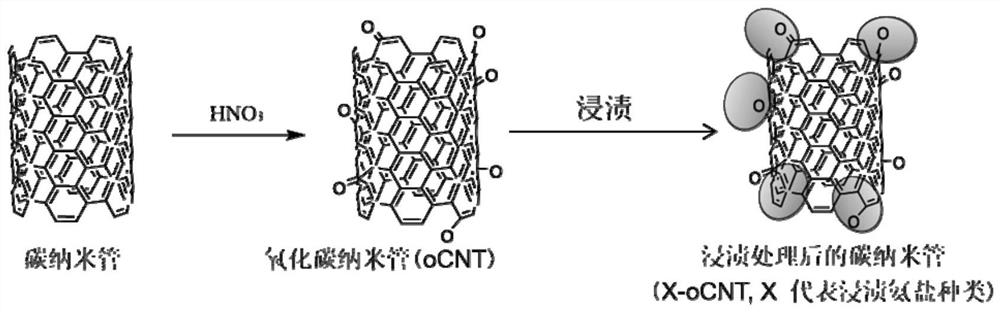
Nano-carbon solid acid catalyst, preparation thereof and application of nano-carbon solid acid catalyst in preparation of olefin by catalytic dehydration of alcohol
A solid acid catalyst, catalytic dehydration technology, applied in catalyst activation/preparation, hydrocarbon production from oxygen-containing organic compounds, physical/chemical process catalysts, etc., can solve problems such as loss of acidic functional groups, poor reaction stability, and easy breakage of dangling bonds , to achieve the effects of increased yield, simple preparation, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
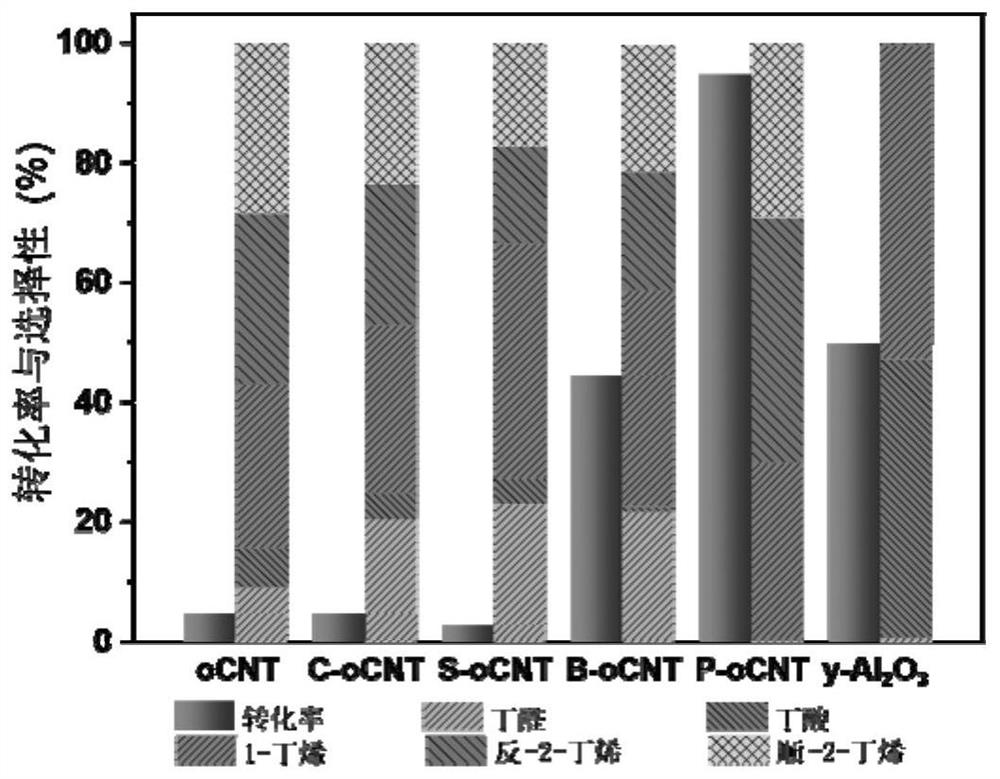
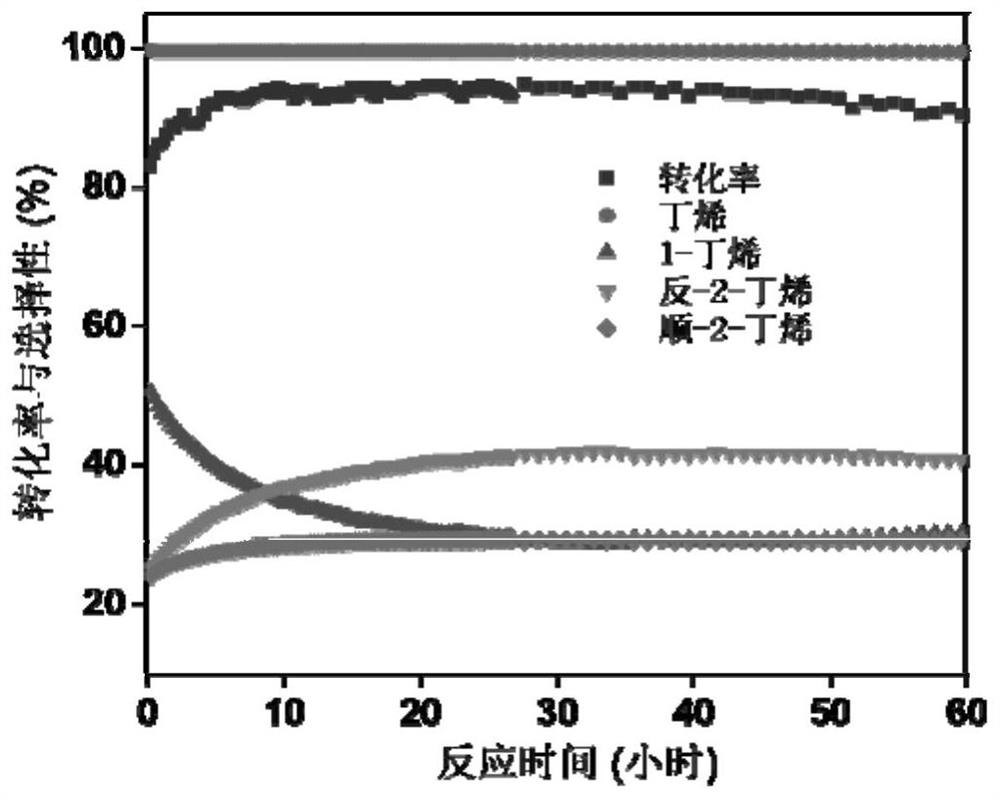
[0050] Get 0.53mL concentration to be 50mg / mL diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) solution, add 0.5g oxidized carbon nanotube (oCNT) in 3mL deionized water, obtain the dispersion liquid of nano carbon material after ultrasonic dispersion; Add the diammonium hydrogen phosphate solution dropwise in the dispersion liquid of nano carbon material under stirring condition , after continuing ultrasonic stirring for 2h, dry in an oven, the drying temperature is 60°C, and the drying time is 12h. After drying, the prepared phosphate solid acid catalyst (P-oCNT) is obtained, and 100mg of P-oCNT catalyst is added to the reaction device. Butanol was introduced at 1KPa, using helium as the carrier gas, and the total flow rate was 18mL / min. Under 260 ℃, carry out reaction, the transformation rate of butanol after reaction is stable is 94%, and the selectivity of butyraldehyde is 99%, as figure 2 shown. And its stability exceeds 60h, such as image 3 shown.
Embodiment 1-2
[0054] Get 1.12mL concentration and be 50mg / mL diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) solution, add 0.5g oxidized carbon nanotube (oCNT) in 2.5mL deionized water, obtain the dispersion liquid of nano carbon material after ultrasonic dispersion; Add the diammonium hydrogen phosphate solution dropwise to the dispersion liquid of nano carbon material under stirring condition , continue ultrasonic stirring for 2 hours, then dry in an oven, the drying temperature is 60°C, and the drying time is 12 hours. After drying, the prepared phosphate solid acid catalyst (2P-oCNT) is obtained, and 100mg of 2P-oCNT catalyst is added to the reaction device. Butanol and oxygen were introduced at 1KPa respectively, using helium as the carrier gas, and the total flow rate was 18mL / min. The reaction was carried out at 260°C. After the reaction was stable, the conversion rate of butanol was 99%, the selectivity of butyraldehyde was 99%, and the stability could be maintained for more than...
Embodiment 1-3
[0056] Add 100 mg of the P-oCNT catalyst prepared in Example 1-1 into the reaction device, pass through 1KPa of butanol, use helium as the carrier gas, and the total flow rate is 18mL / min. Within 200-260°C, the reaction is carried out. Figure 6 shown.
[0057] Figure 7 It is a comparison chart of phosphorus content and catalytic butanol dehydration activity in nano-carbon solid acid catalysts with different phosphate loadings. As shown in the figure, as the load increases, the phosphorus content and catalytic activity on the carbon material surface also increase. When the loading amount is 3-5w%, under this reaction condition, less phosphate can be used to obtain high yield.
[0058] Figure 8 Titration diagram of acid-catalyzed active sites for oxidized carbon nanotubes (oCNTs). As shown in the figure, when the reaction reaches a steady state, the catalytic activity of 2,6-di-tert-butylpyridine gradually decreases with time, indicating that the surface The acidic sit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com