Universal method for preparing metal nitrogen-carbon catalyst material and application of metal nitrogen-carbon catalyst material
A carbon catalyst and metal nitrogen technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of manpower and material resources, complex preparation methods, and difficult control of preparation process conditions. The effect of convenience, wide source of raw materials, and abundant catalytic active sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment prepares the metal nitrogen carbon catalyst material according to the following steps:
[0031] 1) Add 5 g of citric acid monohydrate and 5 g of ammonium chloride into 15 mL of deionized water, sonicate for 10 min, stir at room temperature for 3 h, evaporate the solvent at 70 °C, and dry at 60 °C for 24 h; the obtained product is calcined at 1000 °C for 3 h under argon, A porous nitrogen-carbon material (NC) was obtained.
[0032] 2) Add 14mg of cobalt nitrate hexahydrate into 25mL of ultrapure water, then add 20mg of NC, and sonicate for 1h. Subsequently, an aqueous solution of sodium borohydride (25 mL, 0.25 mol / L) was added dropwise to the mixture, and after the addition was completed, the stirring reaction at room temperature was continued for 12 h; Dry for 12 hours.
[0033] 3) The product obtained in step 2) was calcined at 600° C. for 3 h under argon to obtain the target product metal cobalt nitrogen carbon composite material (Co@NC).
Embodiment 2
[0035] In this example, the metal nitrogen carbon catalyst material was prepared according to the same method as in Example 1, except that the inorganic metal salt cobalt nitrate in step 2) was replaced with nickel nitrate to obtain a metal nickel nitrogen carbon composite material (Ni@NC).
Embodiment 3
[0037] This embodiment prepares the metal nitrogen carbon catalyst material according to the same method as in Example 1, the difference is only: the inorganic metal salt cobalt nitrate in step 2) is replaced with a mixture of cobalt nitrate and nickel nitrate (the molar ratio of the two is 1: 1) to obtain metal cobalt nickel nitrogen carbon composite material (CoNi@NC).
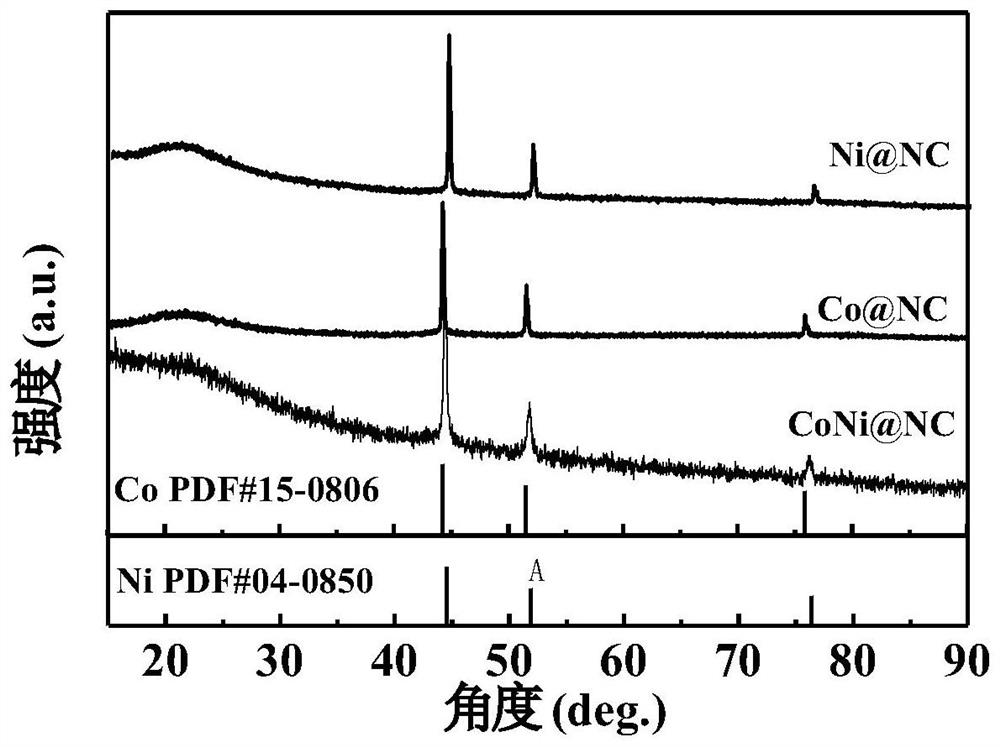
[0038] figure 1 It is the XRD comparison diagram of the metal nitrogen carbon materials prepared in Examples 1-3. It can be seen from the figure that Co@NC, Ni@NC and CoNi@NC composites were successfully prepared.
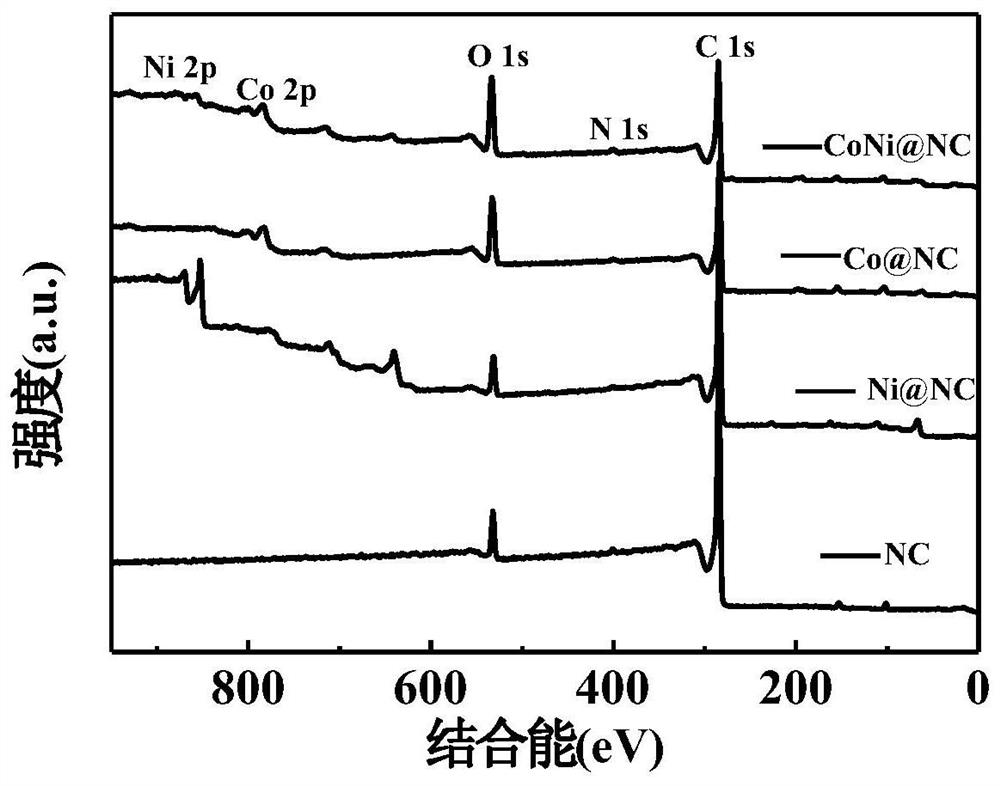
[0039] figure 2 It is the XPS comparison diagram of the metal nitrogen carbon materials prepared in Examples 1-3. It can be seen from the figure that metal elements exist in the corresponding composite materials.
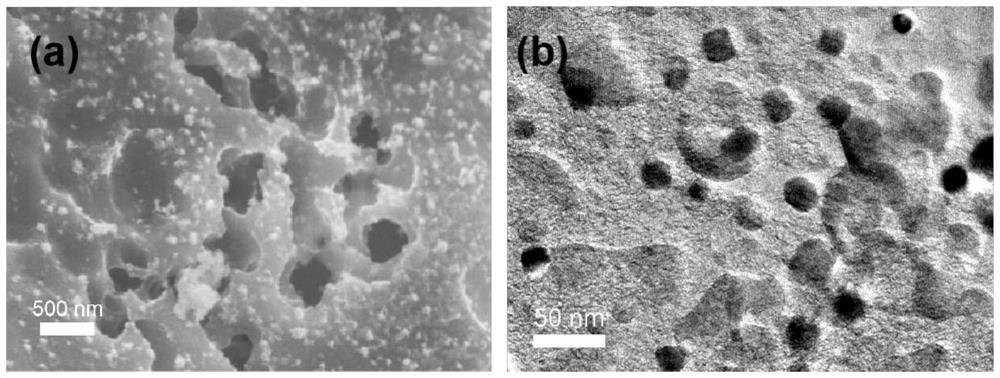
[0040] image 3 The scanning electron micrograph ( image 3 (a)) and transmission electron micrographs ( image 3 (b)). It can be seen from the figure that the cobalt-nickel m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com