Preparation method of 3-hydroxy-4-methoxyphenylpropylaldehyde
A technology of methoxyphenylpropanal and methoxyphenylacrolein, which is applied in the field of organic chemical synthesis, can solve the problems of high price, limited production, and few sources of isovanillin, so as to improve yield and purity, reduce cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
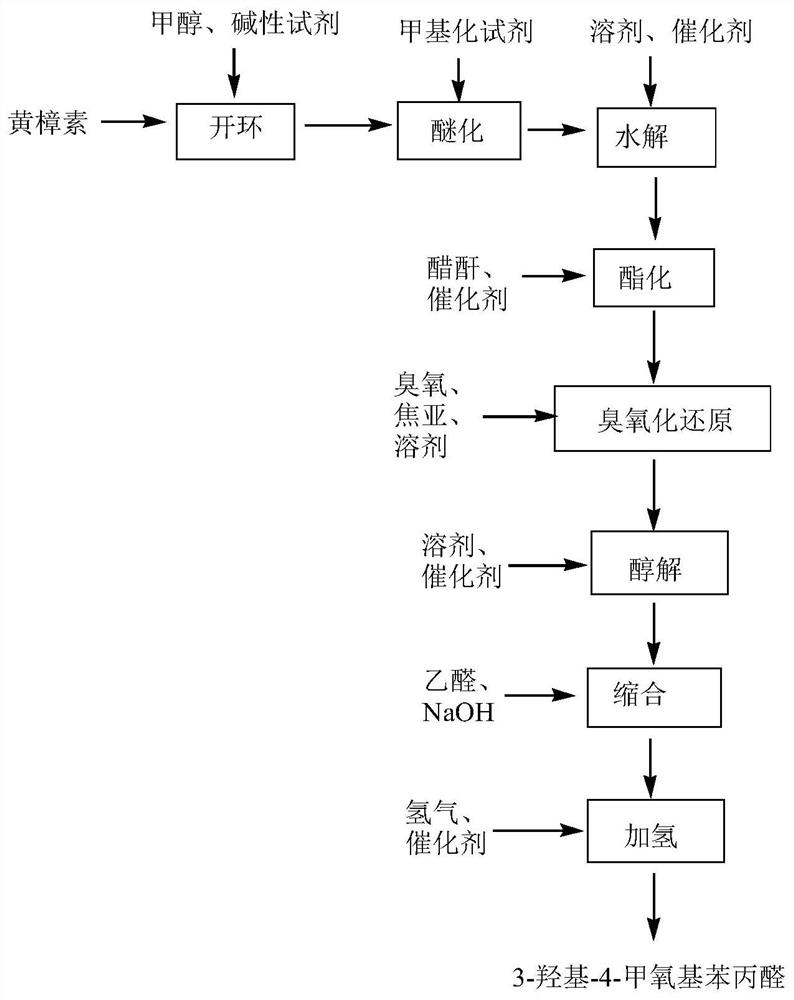
[0049] A preparation method of 3-hydroxyl-4-methoxyphenylpropionaldehyde, comprising the steps of:
[0050] Step 1. Opening the loop: put 100g sassafras oil, 100g methanol, and 80g sodium hydroxide into the reaction kettle, heat up to 60°C after feeding and keep warm for reflux for 0.5h, then distill methanol out under normal pressure, and raise the temperature to 150°C , continue until the content of safrole in the reaction kettle is <1%, stop heating, wait for the reaction solution to cool to 80°C, slowly add 200g of distilled water, mix well, then pour the reaction solution into the separatory funnel, let stand to separate the phases, and separate the lower layer Aqueous phase, obtains potassium phenate solution;
[0051] Step 2. Etherification: Add the potassium phenate solution obtained in Step 1 into the reaction kettle, add excess dimethyl sulfate until the solution is clear and bright, and heat to 20°C for 9 hours, add excess toluene, mix well, and then stand for phase...
Embodiment 2
[0059] A preparation method of 3-hydroxyl-4-methoxyphenylpropionaldehyde, comprising the steps of:
[0060] Step 1. Opening the loop: Put 100g sassafras oil, 100g methanol, and 80g potassium hydroxide into the reaction kettle. After feeding, raise the temperature to 130°C for 5 hours. After the heat preservation and reflux, methanol is distilled out under normal pressure and the temperature is raised. To 160°C, continue until the content of safrole in the reactor <1%, stop heating, wait for the reaction solution to cool to 100°C, slowly add 230g distilled water, mix well, then pour the reaction solution into the separatory funnel, let stand to separate phases, Separating the lower floor water phase to obtain potassium phenate solution;
[0061] Step 2. Etherification: Add the potassium phenate solution obtained in Step 1 into the reaction kettle, add excess methyl trifluoromethanesulfonate until the solution is clear and bright, and heat to 100°C for 1 hour, add excess toluene...
Embodiment 3
[0069] A preparation method of 3-hydroxyl-4-methoxyphenylpropionaldehyde, comprising the steps of:
[0070] Step 1. Opening the loop: Put 100g sassafras oil, 100g methanol, and 80g sodium methoxide into the reaction kettle. After feeding, raise the temperature to 115°C for 3.5 hours. After the heat preservation and reflux, methanol is distilled out under normal pressure and the temperature is raised. To 155°C, continue until the content of safrole in the reactor <1%, stop heating, wait for the reaction solution to cool to 90°C, slowly add 220g of distilled water, mix well, then pour the reaction solution into the separatory funnel, let it stand for phase separation, Separating the lower floor water phase to obtain potassium phenate solution;
[0071] Step 2. Etherification: Add the potassium phenate solution obtained in Step 1 into the reaction kettle, add excess methyl p-toluenesulfonate until the solution is clear and bright, and heat to 75°C for 6 hours, add excess toluene,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com