Flexible porous boron affinity copolymer adsorbent as well as preparation method and application thereof
A technology of copolymers and adsorbents, which is applied in the field of preparation of flexible porous boron affinity copolymer adsorbents, can solve problems such as volume and shape instability, limited applications, flexible porous polymer network structure is easy to disintegrate or collapse, and achieve The effects of excellent chemical properties, simplified adsorption and desorption operations, and excellent mass transfer kinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
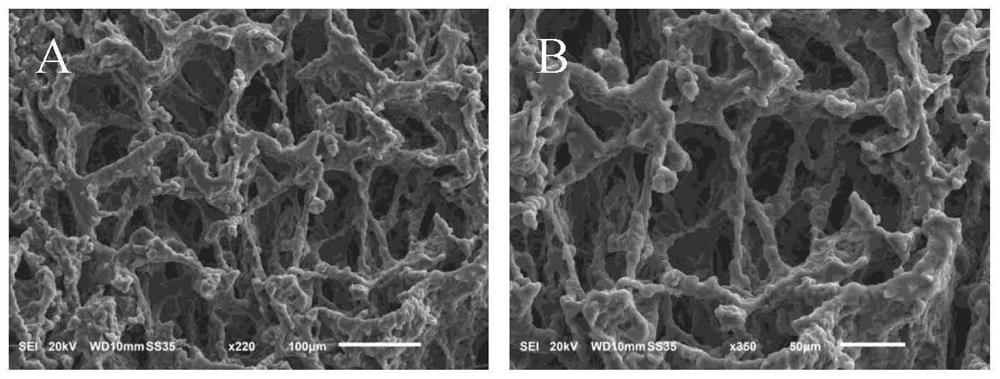
Examples
Embodiment 1
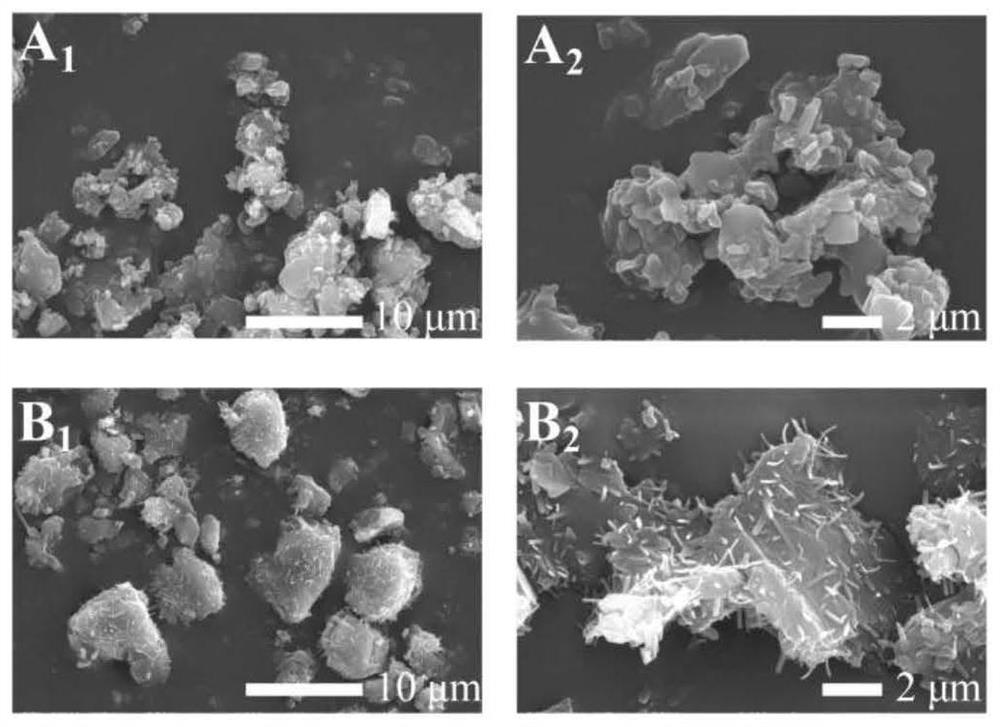
[0038] (1) Preparation of CDPs
[0039] 310mg of p-phenylene diisocyanate and 1g of β-CD were added to 10mL of anhydrous N,N-dimethylformamide solution, and then the reaction system was continuously stirred at 120°C for 20h under the protection of nitrogen. The resulting precipitated product was filtered and washed 3 times with DMF. The final product was further purified by Soxhlet extraction, CDPs-20, and then transferred to a vacuum oven at 80°C for overnight drying to obtain CDPs-20;
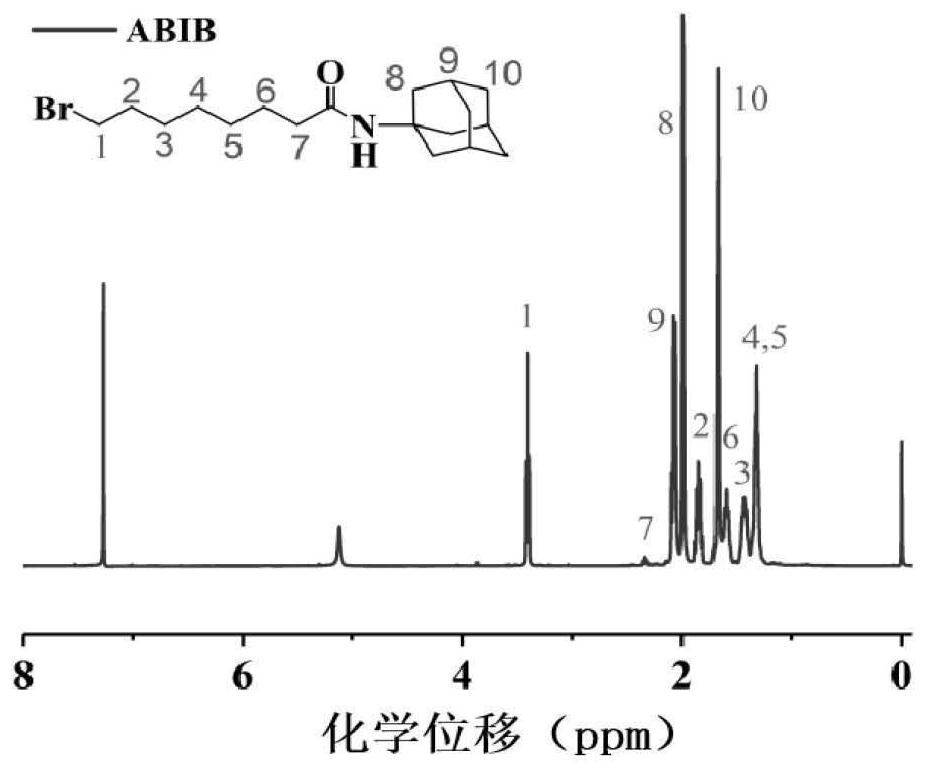
[0040] (2) Synthesis of brominated adamantane polymer (ABIB)
[0041]First, at room temperature, 2.2 g of 6-bromohexanoic acid was dissolved in 9 ml of anhydrous chloroform, and then 25 ml of thionyl chloride was added dropwise to the above solution within 30 minutes, The above solution was stirred and reacted for 20 hours under the protection of nitrogen, and finally the excess thionyl chloride was removed by rotary evaporation to finally obtain the intermediate product ABIB.
[0042] (3)...
Embodiment 2
[0051] (1) Preparation of CDPs
[0052] 320mg of p-phenylene diisocyanate and 1.1g of β-CD were added to 20mL of anhydrous N,N-dimethylformamide solution, and then the reaction system was continuously stirred at 120°C for 20h under the protection of nitrogen. The resulting precipitated product was filtered and washed 3 times with DMF. The final product was further purified by Soxhlet extraction, CDPs-20, and then transferred to a vacuum oven at 80°C for overnight drying to obtain CDPs-20;
[0053] (2) Synthesis of brominated adamantane polymer (ABIB)
[0054] First, at room temperature, 2.22 g of 6-bromohexanoic acid was dissolved in 10 ml of anhydrous chloroform, and then 30 ml of thionyl chloride was added dropwise to the above solution within 30 minutes, The above solution was stirred and reacted for 20 hours under the protection of nitrogen, and finally the excess thionyl chloride was removed by rotary evaporation to finally obtain the intermediate product ABIB.
[0055...
Embodiment 3
[0060] (1) Preparation of CDPs
[0061] 330mg of p-phenylene diisocyanate and 1.2g of β-CD were added to 30mL of anhydrous N,N-dimethylformamide solution, and then the reaction system was continuously stirred at 120°C for 20h under the protection of nitrogen. The resulting precipitated product was filtered and washed 3 times with DMF. The final product was further purified by Soxhlet extraction, CDPs-20, and then transferred to a vacuum oven at 80°C for overnight drying to obtain CDPs-20;
[0062] (2) Synthesis of brominated adamantane polymer (ABIB)
[0063] First, at room temperature, 2.24 g of 6-bromohexanoic acid was dissolved in 11 ml of anhydrous chloroform, and then 35 ml of thionyl chloride was added dropwise to the above solution within 30 minutes, The above solution was stirred and reacted for 20 hours under the protection of nitrogen, and finally the excess thionyl chloride was removed by rotary evaporation to finally obtain the intermediate product ABIB.
[0064...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com