Synthesis method of 2-isopropyl-3-amino-4-methylpyridine
A kind of methyl pyridine, synthetic method technology, applied in the direction of organic chemistry etc., can solve the problem such as not giving post-treatment mode, reaction yield and purity, L-proline catalytic effect is poor, environment is not friendly, reach product The effect of clean production, environmental friendliness and reduction of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
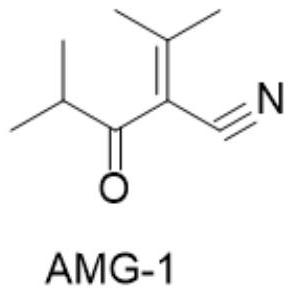
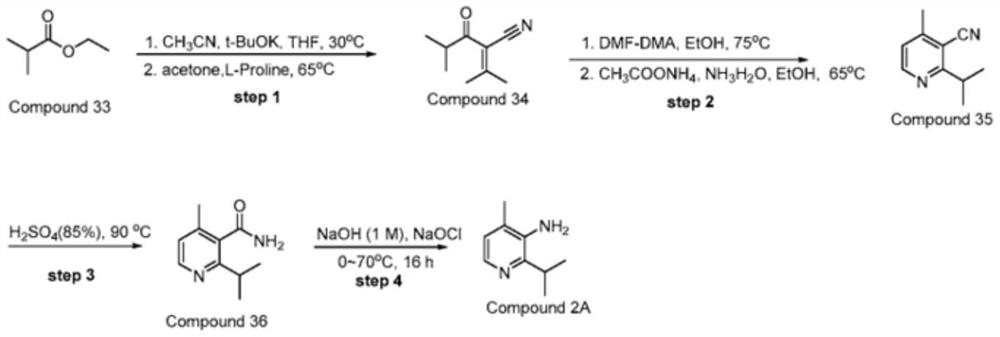
[0042] Synthesis of 2-isobutyryl-3-methyl-2-butenenitrile: In a four-necked flask equipped with a thermometer and a stirrer, add 11.6g of ethyl isobutyrate, 60ml of THF and 20g of potassium tert-butoxide, and start stirring , control the reaction temperature at 45-50°C, add 70g of acetonitrile dropwise, and keep the temperature for 4 hours. After the reaction of the raw materials, cool down to below 10°C, then add glacial hydrochloric acid to adjust the pH to 3-4, filter, and spin dry the organic phase to obtain 11.1g of crude product .
[0043] In a four-necked flask equipped with a thermometer and a stirrer, add 11.1g of the crude product obtained above, 80ml of acetone, 100g of 4A molecular sieve and 1.11g of L-alanine, start stirring, raise the temperature to reflux for reaction, keep the temperature for 4 hours, and the reaction of the raw materials is complete Finally, filter and recover 4A molecular sieve, evaporate the organic phase to dryness and recover a...
Embodiment 2
[0055]
[0056] Synthesis of 5-N,N-dimethyl-2-isobutyryl-2,4-dipentenenitrile: Add 15.1g of 2-isobutyryl-3- Methyl-2-butenenitrile, 60ml ethanol and 11.9g DMF-DMA, start stirring, heat up to reflux reaction, keep warm for 3h, after the reaction of the raw materials is completed, evaporate the solvent, add 50ml water for beating, filter, and dry to obtain 5 -N,N-Dimethyl-2-isobutyryl-2,4-dipentenenitrile 19.1 g, purity 99.5%, yield 93%.
Embodiment 3
[0058]
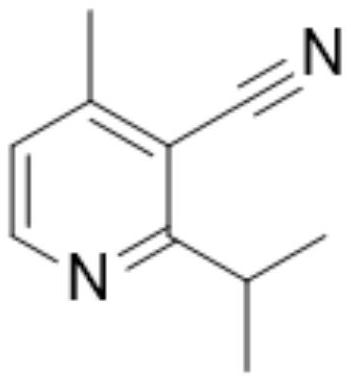
[0059] Synthesis of 2-isopropyl-4-methyl-3-cyanopyridine: Add 20.6g of 5-N,N-dimethyl-2-isobutyryl- 2,4-dipentenenitrile, 80ml of ethanol and 30.8g of ammonium acetate, start stirring, raise the temperature to reflux reaction, keep warm for 6h, after the reaction of raw materials, evaporate the solvent, add 50ml of water to wash, and extract with dichloromethane, separate the liquid , spin-dried to recover dichloromethane to obtain 12.5 g of 2-isopropyl-4-methyl-3-cyanopyridine with a purity of 99.3% and a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com