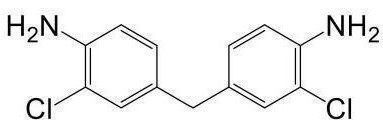
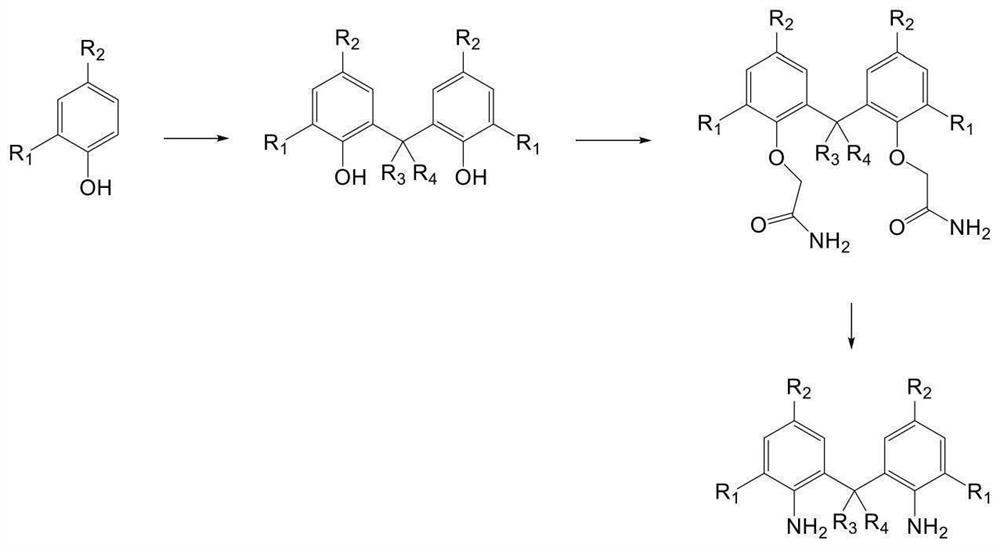
Lignin-based polyurethane chain extender as well as preparation method and application thereof
A lignin-based and polyurethane technology, applied in the direction of biological raw materials, can solve the problem of browning, etc., and achieve the effect of simple operation, reduced dependence, and enhanced thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070]
[0071] IIIa 2,2'-methylene (4-methylphenol)
[0072] Accurately weigh 4-methylphenol (21.6 g, 0.2 mol), 40% formaldehyde solution (9.0 g, 0.12 mol) and p-toluenesulfonic acid (1.72 g, 0.01 mol) in the pressure resistant bottle, 60 ° C water bath heating After stirring for 30 minutes, the reaction was diluted with ethyl acetate, water and ethyl acetate were extracted, dried off in anhydrous sulfate, and the organic phase was concentrated, and the viscous oil liquid (Compound IIIA) was obtained, and the yield was 80.3%. MSI-MS: 229.3 [M + H] + .
[0073] IVA 2,2 '- (((methylene (4-methyl-2,1-phenyl)) bis ((oxygen)) double acetamide
[0074] Accurately weigh IIIa (11.4 g, 0.05 mol), chloroacetamide (5.8 g, 0.0625 mol), potassium anhydrous carbonate (15.5 g, 0.1125 mol), potassium iodide (0.83 g, 0.005 mol) in a 2L round bottom flask, 1.25L acetone, 60 ° C for 6 h, after the end of the reaction, ol drying, water and ethyl acetate extraction, dried anhydrous sulfate, and con...
Embodiment 2
[0078]
[0079] 2A 6,6'-methylene (2,4-dimethylphenol)
[0080] Referring to IIIA synthesis, 2,4-dimethylphenol replace 4-methylphenol, yield of 80.5%. MSI-MS: 257.4 [M + H] + .
[0081] 2B 2, 2 '- (((methylene (4,6-dimethyl-2,1-phenyl)) bis ((oxygen)) double acetamide
[0082] The yield is 98.9% with reference to the IVA synthesis method. MSI-MS: 371.5 [M + H] + .
[0083] I-2 6,6'-methylene (2,4-dimethyl ketaniline)
[0084] Referring to I-1 synthesis method, the yield is 98.4%. MSI-MS: 255.4 [M + H] + .
Embodiment 3
[0086]
[0087] 3A 6,6'-methylene (2-methoxy-4-methylphenol)
[0088] Referring to IIIa synthesis, 4-methyl-2-methoxyphenol replace 4-methylphenol, yield of 79.2%. MSI-MS: 289.3 [M + H] + .
[0089] 3B 2, 2 '- (((methylene (6-methoxy-4-methyl-2,1-phenyl)) bis ((oxygen)) double acetamide
[0090] The yield is 96.4% with reference to the IVA synthesis method. MSI-MS: 403.4 [M + H] + .
[0091] I-3 6,6'-methylene (2-methoxy-4-methylhniline)
[0092] Referring to I-1 synthesis, the yield is 97.6%. MSI-MS: 287.3 [M + H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com