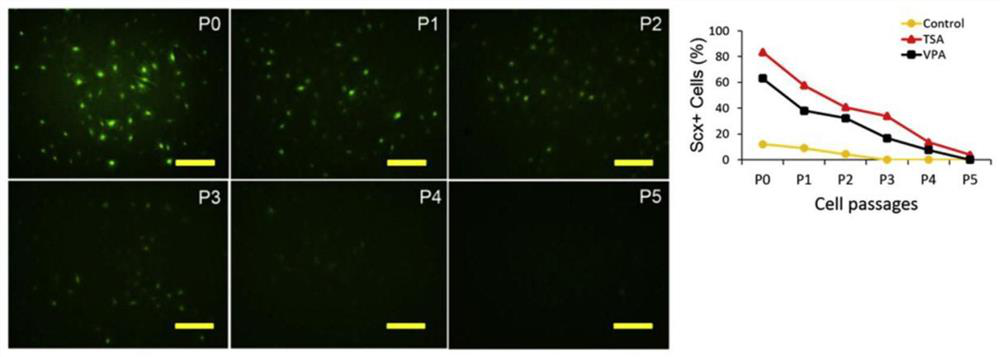
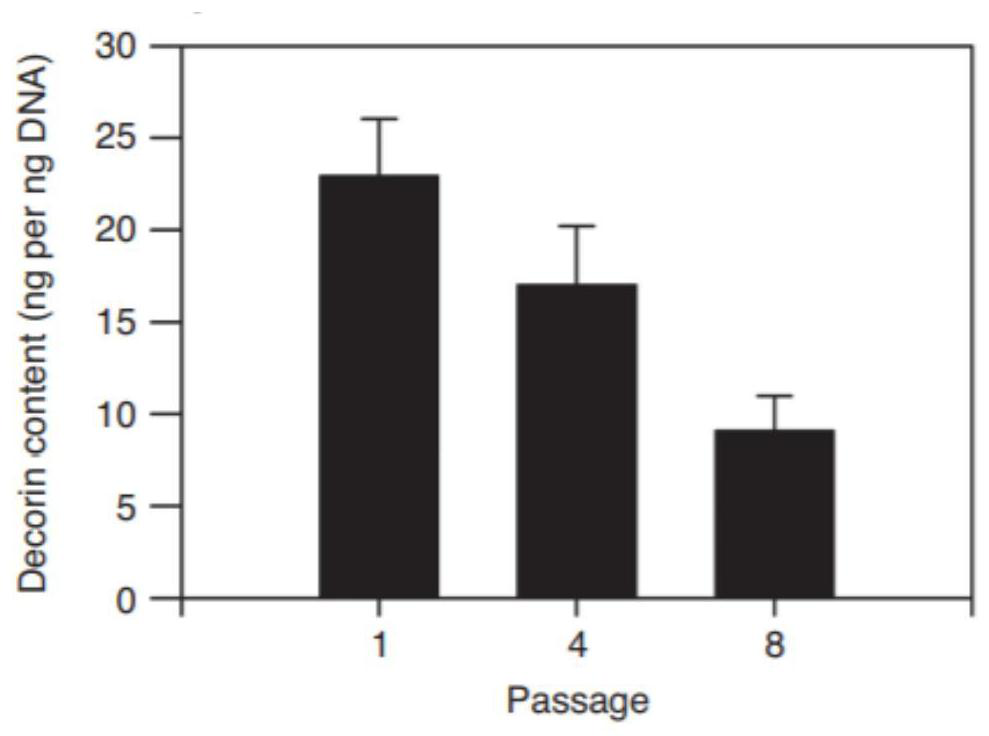
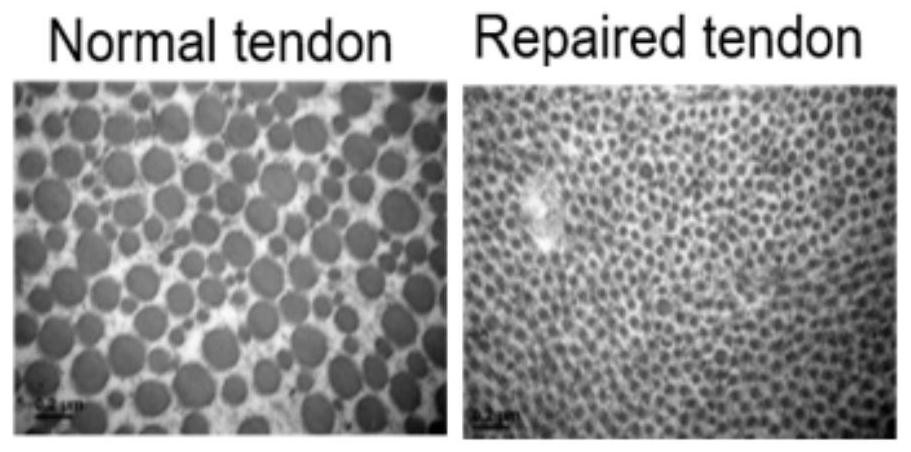
Bioactive substance composition, serum-free culture medium containing composition and application of serum-free culture medium
A technology of biologically active substances and compositions, applied in the field of biomedicine, can solve the problems of slow cell proliferation, failure of damage repair, and small number, and achieve the effects of good cell growth, clear components and unique proportions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] In this example, two groups of medium were prepared, namely: serum-free medium and serum control medium, and the cultured cells were tendon stem cells (hTSPCs) extracted from human normal tendon tissue. The following are detailed experiments and tests step:
[0179] Medium configuration
[0180] Serum-free medium (SFM)
[0181] The serum-free medium includes basal medium and additional components; the basal medium is selected from DMEM / F12 medium, and 5mmol of HEPES, 10000U of penicillin and 10000U of streptavidin are added in every 500mL of DMEM / F12 medium and add additional components so that the concentration range ratio of each component in the serum-free medium is: fibroblast growth factor: platelet-derived growth factor: transforming growth factor-β: glucocorticoid: heparin or its salt: Vitamin C or its derivatives: transferrin: insulin: progesterone: putrescine or its salts: selenite: epidermal growth factor: CHIR99021=10:10:5:2:1000:25000:2000:500:2 :500:2:10...
Embodiment 2
[0259] In this example, two groups of medium were prepared, namely: serum-free medium and serum control medium, and the cultured cells were ligament stem cells obtained by separating and culturing human ligament tissue. The following are the detailed experimental and detection steps:
[0260] Medium configuration
[0261] Serum-free medium (SFM)
[0262] The serum-free medium includes basal medium and additional components; the basal medium is selected from F10 medium, and 5mmol of HEPES, 10000U of penicillin and 10000U of streptomycin are added to each 500mL of F10 medium, and Add components so that the concentration range ratio of each component in the serum-free medium is: fibroblast growth factor: platelet-derived growth factor: transforming growth factor-β: glucocorticoid: heparin or its salt: vitamin C or its Derivatives: transferrin: insulin: progesterone: putrescine or its salt: selenite=15:10:6:2:1500:25000:75000:3000:4:7:4.
[0263] Further, the concentration of ea...
Embodiment 3
[0276] In this example, two groups of medium were prepared, namely: serum-free medium and serum control medium, and the cultured cells were tendon stem cells (hTSPCs) extracted from human normal tendon tissue. The following are detailed experiments and tests step:
[0277] Medium configuration
[0278] Serum-free medium (SFM)
[0279] The serum-free medium includes basal medium and additional components; the basal medium is selected from DMEM / F12 medium, and 5mmol of HEPES, 10000U of penicillin and 10000U of streptavidin are added in every 500mL of DMEM / F12 medium element, and add additional components so that the concentration of the additional components in the serum-free medium is: fibroblast growth factor: platelet-derived growth factor: transforming growth factor-β: glucocorticoid: heparin or its salt: vitamin C or its derivatives: transferrin: insulin: progesterone: putrescine or its salt: selenite=50:50:40:11:1000:100000:300000:25000:25:25000:25; The medium also cont...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com