Propiconazole hapten as well as preparation method and application thereof
A technology of propiconazole and hapten, which is applied in the biological field, can solve the problems of expensive instruments and cannot be widely used, and achieve the effects of rapid detection, good affinity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
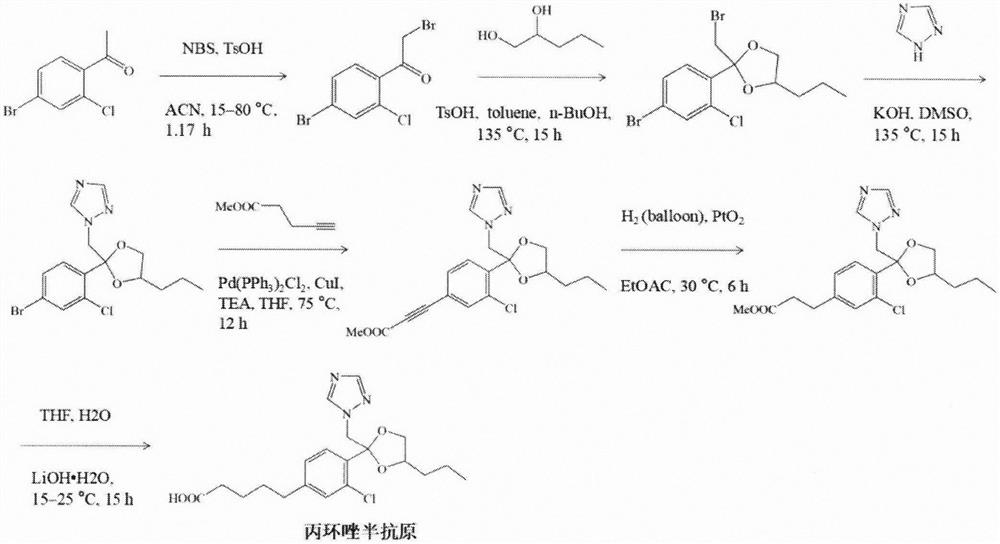
[0038] Example 1: Synthesis of propiconazole hapten
[0039] Step 1) Add 1.52g of N-bromosuccinimide and 2g of 2-chloro-4-bromo-acetophenone into 12mL of acetonitrile, and stir the reaction at 12°C for 10 minutes. Then 2.95 g of p-toluenesulfonic acid was added to the above mixture, and the mixture was stirred and reacted at 15-80° C. for 1.17 hours. The product obtained by the reaction was purified by a silica gel column, eluted and separated with petroleum ether-ethyl acetate at a volume ratio of 100:1 to 10:1, and product 1 was obtained.
[0040] Step 2) 4g of product 1 and 2g of 1,2-pentanediol were added to 24mL of toluene, then 12mL of n-butanol and 1.1g of p-toluenesulfonic acid were added, and the above mixture was stirred and reacted at 135°C for 15h. After the reaction, the product was purified through a silica gel column and eluted with petroleum ether-ethyl acetate at a volume ratio of 100:1 to 10:1 to obtain product 2.
[0041] Step 3) 1.39g KOH and 0.73g 1H-1,2,4...
Embodiment 2
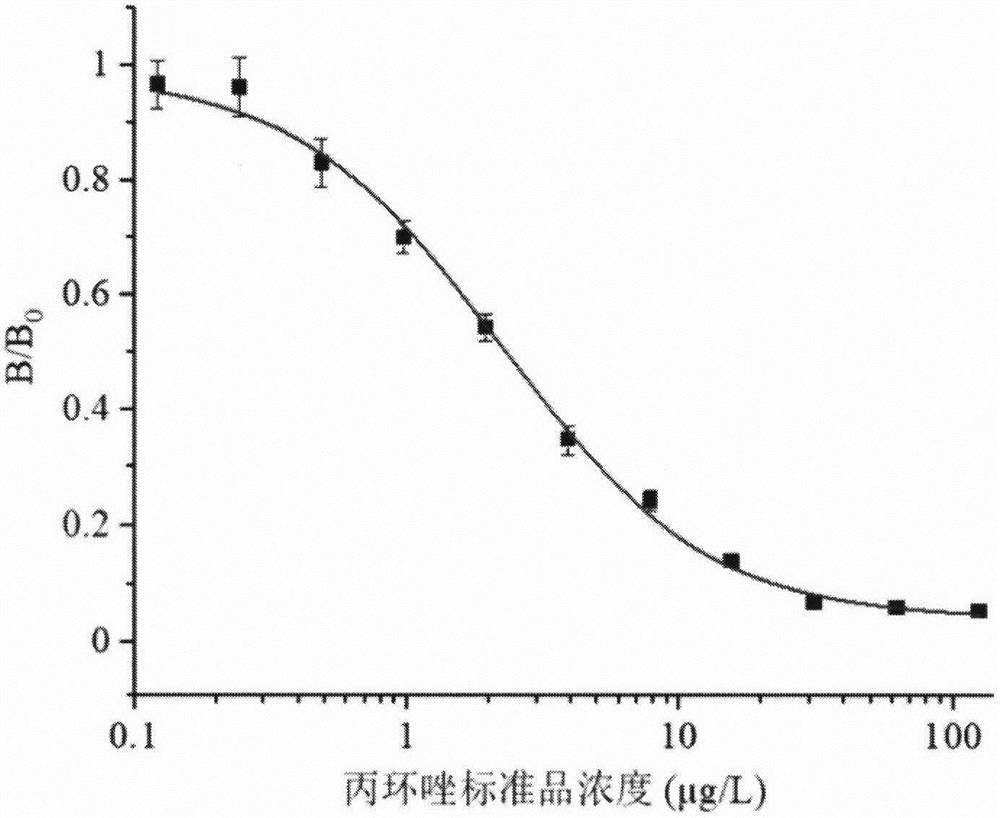
[0045] Example 2: Identification of propiconazole hapten
[0046] The results of NMR identification of propiconazole hapten: 1H NMR (500MHz, DMSO-d6) δ8.36(d, J=7.1Hz, 1H), 7.84(d, J=16.3Hz, 1H), 7.41(dd, J =14.9, 7.9Hz, 1H), 7.31(dd, J=6.9, 1.6Hz, 1H), 7.13(ddd, J=18.7, 8.0, 1.7Hz, 1H), 4.77-4.6(m, 2H), 3.91- 3.75(m, 2H), 3.15(q, J=6.6, 5.5Hz, 1H), 2.58(td, J=7.5, 3.3Hz, 2H), 2.23(t, J=7.3Hz, 2H), 2.07(s , 1H), 1.62-1.46 (m, 4H), 1.27 (tdt, J=25.7, 14.3, 7.1 Hz, 4H), 0.92-0.72 (m, 3H).
Embodiment 3
[0047] Embodiment 3: the preparation of propiconazole antigen
[0048] Step 1) Activation of hapten. Get 0.082g propiconazole hapten as claimed in claim 1 and dissolve in 1.5mL N,N-dimethylformamide, after adding 0.023g N-hydroxysuccinimide to the above solution, stir at room temperature for 15 minutes , and then 1 mL of N,N-dimethylformamide solution containing 0.041 g of N,N-dicyclohexylcarbodiimide was added to the mixture, and the reaction was stirred overnight.
[0049] Step 2) Coupling of the hapten to the carrier protein. Centrifuge the reaction product in step 1 at 4000 rpm for 5 minutes, take 1.25 mL of the supernatant and slowly add it dropwise to 5 mL of 10 mg / mL bovine serum albumin in phosphate buffer (0.01 mol / L, pH=8), and stir at room temperature in the dark After reacting for 4 hours, propiconazole immune antigen was obtained; another 1.25 mL of centrifuged product in step 1 was slowly added dropwise to 5 mL of 10 mg / mL chicken ovalbumin phosphate buffer (0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com