Bromination reaction method of nitrogen-containing heterocyclic compound and application
A nitrogen heterocyclic compound, bromination reaction technology, applied in the direction of organic chemistry, can solve the problem of debromination products, etc., to achieve the effect of good yield, high selectivity, and good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
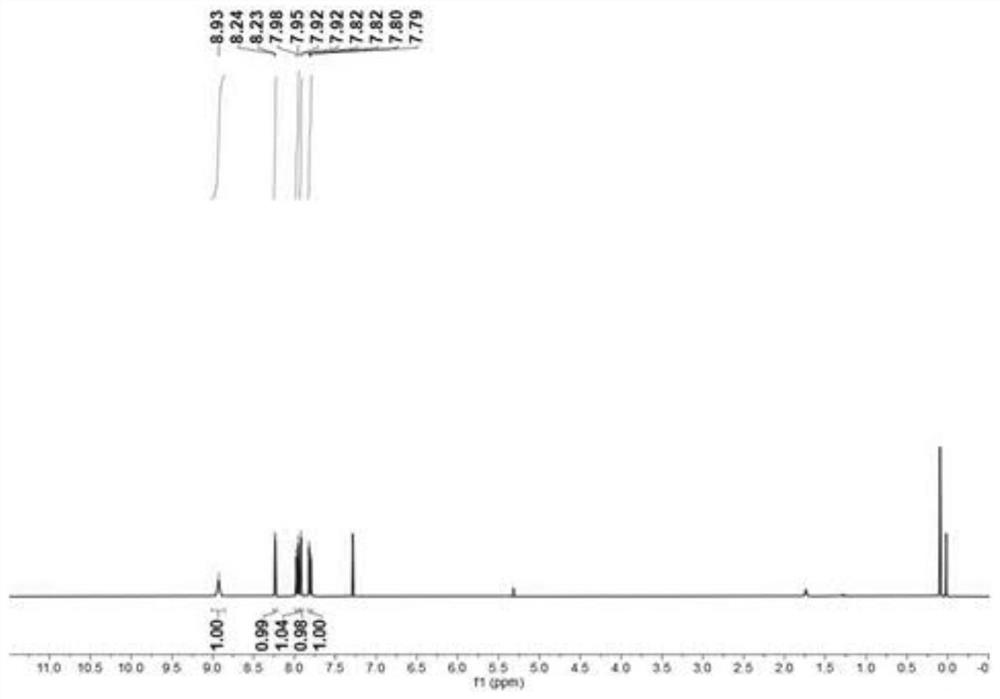
Embodiment 1
[0049] This embodiment provides a method for the bromination reaction of nitrogen-containing heterocyclic compounds, the reaction formula of which is:
[0050]
[0051] The specific operation steps are:
[0052] In air, add magneton, N-benzyl-5-nitroquinoline quaternary ammonium salt I-a (0.1mmol, 29.9mg), N-bromosuccinimide (0.2mmol , 34.8mL), benzoic acid (0.05mmol, 6.1mg), dichloroethane (1.5mL). Then put on a rubber stopper and heat and stir in an oil bath at 100°C for 4h. After the reaction was completed, the reaction system was cooled to room temperature, filtered with a glass sand funnel lined with diatomaceous earth, washed with dichloromethane and ethyl acetate, combined the filtrates, evaporated the solvent under reduced pressure, added 10 mL of distilled water, and then used 30 mL The ethyl acetate is divided into 3 extractions, and the extract is dried with anhydrous magnesium sulfate, and the solvent is evaporated under reduced pressure, and petroleum ether / eth...
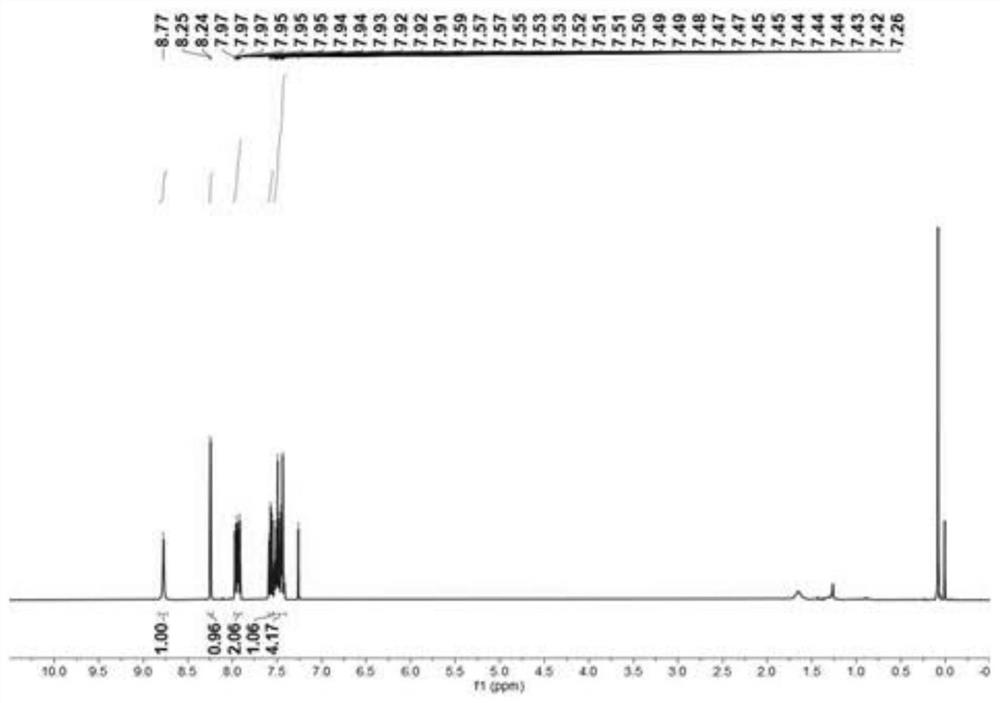
Embodiment 2
[0056] This embodiment provides a method for the bromination reaction of nitrogen-containing heterocyclic compounds, the reaction formula of which is:
[0057]
[0058] The specific operation steps are:
[0059] In air, to a 25 mL reaction tube was added magneton, N-benzyl-2-(naphthyl-1-yl)pyridinium quaternary ammonium salt I-c (0.1 mmol, 37.5 mg), N-bromosuccinyl Imine (0.2mmol, 34.8mL), p-fluorobenzoic acid (0.05mmol, 6.1mg), dichloroethane (1.5mL). Then put on a rubber stopper and heat and stir in an oil bath at 100°C for 4h. After the reaction was completed, the reaction system was cooled to room temperature, filtered with a glass sand funnel lined with diatomaceous earth, washed with dichloromethane and ethyl acetate, combined the filtrates, evaporated the solvent under reduced pressure, added 10 mL of distilled water, and then used 30 mL The ethyl acetate was extracted three times, the extract was dried with anhydrous magnesium sulfate, the solvent was evaporated u...
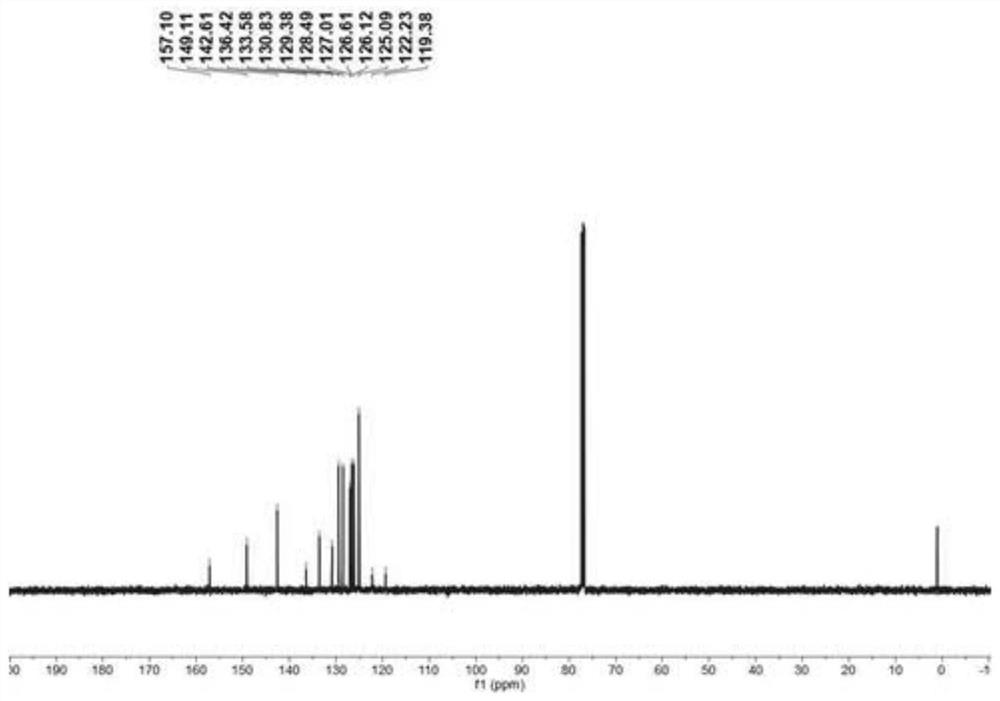
Embodiment 3
[0066] This embodiment provides a method for the bromination reaction of nitrogen-containing heterocyclic compounds, the reaction formula of which is:
[0067]
[0068] The specific operation steps are:
[0069] In air, add magneton, N-benzyl-2-phenylpyridinium quaternary ammonium salt I-d (0.1mmol, 32.5mg), N-bromosuccinimide (0.2mmol, 34.8mL), 2-fluorobenzoic acid (0.05mmol, 7.0mg), dichloroethane (1.5mL). Then put on a rubber stopper and heat and stir in an oil bath at 100°C for 16h. After the reaction was completed, the reaction system was cooled to room temperature, filtered with a glass sand funnel lined with diatomaceous earth, washed with dichloromethane and ethyl acetate, combined the filtrates, evaporated the solvent under reduced pressure, added 10 mL of distilled water, and then used 30 mL The ethyl acetate was extracted three times, the extract was dried with anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, petroleum ether / ethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com