Method for efficiently extracting lithium from lithium-rich aluminum electrolyte waste residues and preparing anhydrous aluminum fluoride
A technology of aluminum electrolyte and water aluminum fluoride, applied in aluminum fluoride, chemical instruments and methods, aluminum halide, etc., can solve the problem of ineffective utilization and other problems, and achieve the effect of thorough removal, efficient recovery and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
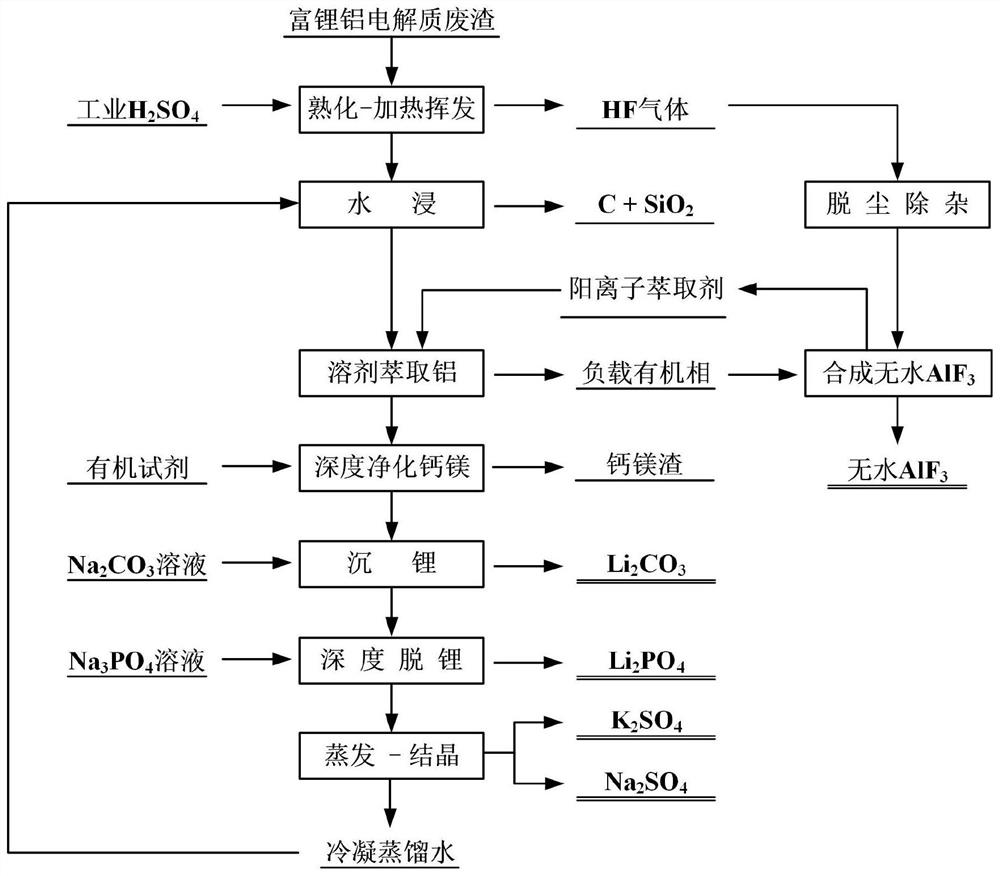
[0085] Take 100g of lithium-rich aluminum electrolyte waste residue, 136.23g of 98% concentrated sulfuric acid, mix and mature for 10min, and then heat at 275°C for 1.5h to obtain defluorinated material and hydrogen fluoride gas. The fluorine removal rate is 99.51%.
[0086] Mix the obtained defluorinated material with water, the ratio of defluorinated material to water is 1g:5mL, then stir at 80°C at 500rpm for 2h; after stirring, filter and separate to obtain primary filtrate and primary filter residue, Li, Na , the leaching rate of K is greater than 99.50%, the leaching rate of Al is 98.46%, and the leaching residue is mainly C, SiO 2 , CaSO 4 .
[0087] Prepare the extraction phase, the extraction agent is diethylhexyl phosphate, the diluent is sulfonated kerosene, the volume ratio of the extraction agent and the extraction phase is 0.2; the primary filtrate and the extraction phase are mixed according to the volume of 1:1, and the pH value is adjusted to 4.3 Carry out e...
Embodiment 2
[0095] Take 100g of lithium-rich aluminum electrolyte waste residue, 142.71g of 98% concentrated sulfuric acid, mix and mature for 30min, and then heat at 250°C for 2h to obtain defluorinated material and hydrogen fluoride gas. The fluorine removal rate is 99.23%.
[0096] Mix the obtained defluorinated material with water, the ratio of defluorinated material to water is 1g:7mL, then stir at 60°C at 450rpm for 3h; after stirring, filter and separate to obtain primary filtrate and primary filter residue, Li, Na , the leaching rate of K is greater than 99.12%, the leaching rate of Al is 98.18%, and the leaching residue is mainly C, SiO 2 , CaSO 4 .
[0097] Prepare the extraction phase, the extraction agent is dioctylphenyl phosphate, the diluent is sulfonated kerosene, the volume ratio of the extraction agent and the extraction phase is 0.25; the primary filtrate and the extraction phase are mixed according to the volume of 2:1, and the pH value is adjusted Extraction was car...
Embodiment 3
[0104] Take 100g of lithium-rich aluminum electrolyte waste residue, 149.2g of 98% concentrated sulfuric acid, mix and mature for 60min, and then heat at 200°C for 3h to obtain defluorinated material and hydrogen fluoride gas. The fluorine removal rate is 99.83%.
[0105] Mix the obtained defluorinated material with water, the ratio of defluorinated material to water is 1g:9mL, then stir at 80°C at 550rpm for 4h; after stirring, filter and separate to obtain the primary filtrate and primary filter residue, Li, Na , the leaching rate of K is greater than 99.75%, the leaching rate of Al is 99.31%, and the leaching residue is mainly C, SiO 2 , CaSO 4 .
[0106] Prepare the extraction phase, the extraction agent is diethylhexyl phosphate, the diluent is sulfonated kerosene, the volume ratio of the extraction agent and the extraction phase is 0.3; the primary filtrate and the extraction phase are mixed according to the volume of 3:1, and the pH value is adjusted to 3.2 Carry out ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com