Application of quinacrine in preparation of medicine for treating pulmonary fibrosis
A pulmonary fibrosis and drug technology, applied in the field of chemical drug development, can solve the problem of lack of specific and effective treatment methods, and achieve the effect of broadening the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
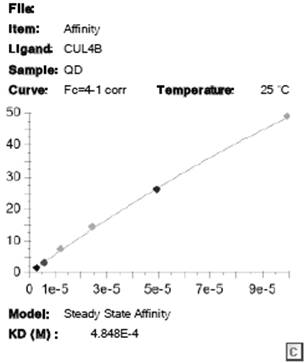
[0040] Surface Plasmon Resonance Technology Experiment (Surface Plasmon Resonance SPR Experiment)
[0041] 1) Sample information
[0042] Stationary Phase:
[0043] name molecular weight Buffer concentration CUL4B 130.4 KD Tris 30 μg / mL
[0044] mobile phase:
[0045] name molecular weight Buffer (quinacrine hydrochloride content) quinacrine hydrochloride 472.88 65mg
[0046] 2) Instrument parameters
[0047] Protein immobilization: Instrument: Biacore S200; chip type: S-CM5; Running buffer: PBS-P; Ligand concentration: 30 μg / mL; flow rate: 10 μL / min. Regeneration conditions: regeneration solution: 10 mM glycine-HCl, pH 2.0; injection time: 30 s; flow rate: 30 μL / min. Kinetic analysis: Control channel: flow cell 1; Running buffer: PBSP, 2%DMSO; Contact time: 120 s; Dissociation time: 300 s; Flow rate: 30 μL / min;
[0048] 3) Experimental process
[0049] Sample dissolution and dilution:
[0050] 3.1) Liquid exchange...
Embodiment 2
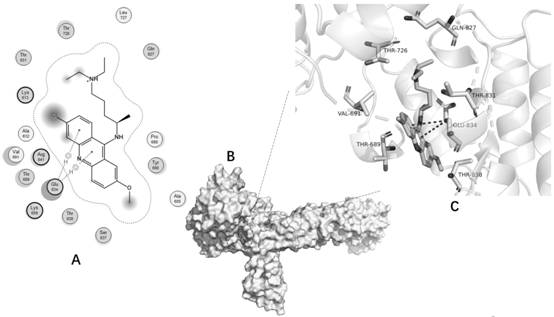
[0075] Computer Simulation Molecular Docking Experiment (MOE-Dock Experiment)
[0076] MOE Dock was used for molecular docking of quinacrine hydrochloride and CUL4B. The 2D structure of quinacrine was downloaded from PubChem and converted to a 3D structure in MOE by energy minimization as a ligand. The crystal structure of CUL4B was downloaded from the RCSB protein database (http: / / www.rcsb.org / ), PDB ID is 4A0C2. The C chain of 4A0C is used as the pair acceptor. Before docking, AMBER10: Implicit solvation model of force field and reaction field (r-field) of EHT was selected. Select the "Induced Fit" scheme to allow the receptor binding site side chains to move according to the ligand conformation and impose constraints on their position. The weight used to tie the side chain atoms to their original positions was 10. First, the London dG scoring function is used to score all docking poses, and then the force field refinement is performed on the first 30 poses, and then the...
Embodiment 3
[0081] Drug (quinacrine) and target protein (CUL4B) binding stability test (DARTS test)
[0082] 1) Plasmid construction and transfection: Plasmid construction is the most commonly used experimental technique in molecular biology research. The principle relies on the action of restriction endonuclease, DNA ligase and other modifying enzymes. After appropriate cutting and modification of the target gene and carrier DNA, the two are connected together and then introduced into the host cell to realize the target gene in the host cell. Correct expression in host cells. Plasmids containing different CUL4B protein domains with FLAG tags were constructed respectively, and the constructed plasmids were respectively transfected into tool cell 293T cells and then incubated at 37°C and 5% CO 2 and cultured in an incubator with saturated humidity for 48 h.
[0083] 2) Prepare DARTS cell lysate (1 mL): M-PER mammalian protein extraction reagent 730 μL, protease inhibitor 10 μL, 200 mM ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com