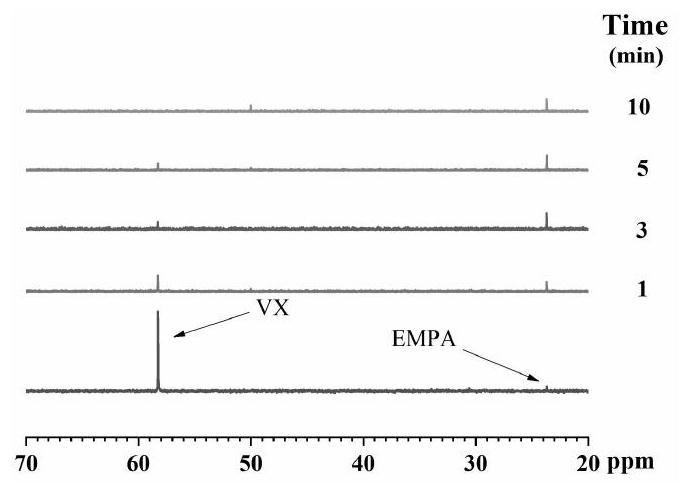
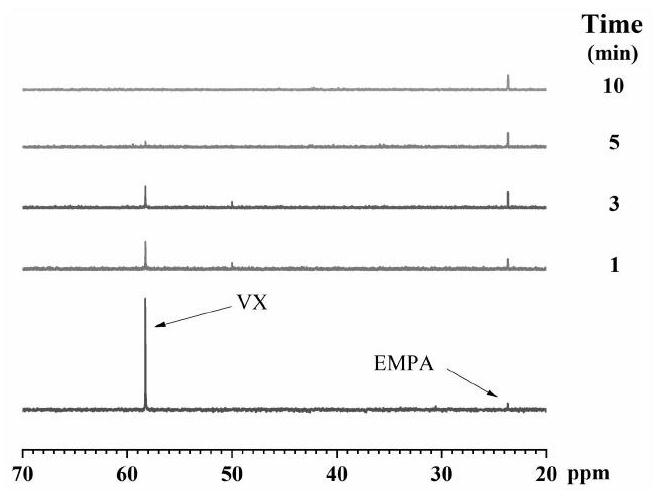
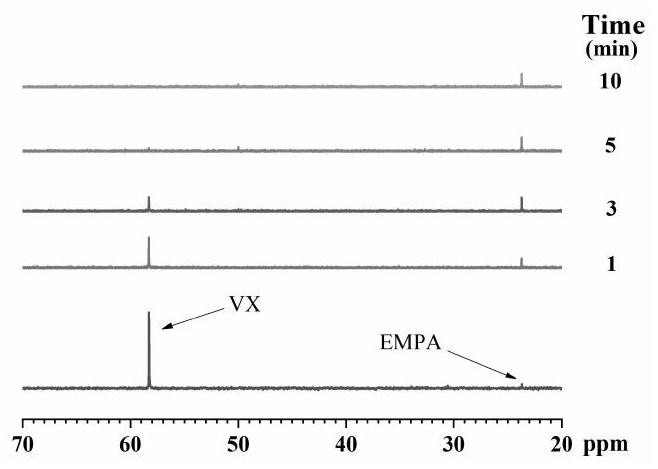
Chemical warfare agent digestion material and preparation method of fiber material modified by chemical warfare agent digestion material
A chemical warfare agent and fiber material technology, applied in the field of chemical warfare agent digestion and protection materials, can solve the problems of long time, easy deactivation, long time, etc., and achieve the effects of mild reaction conditions, rapid digestion ability, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation method of chemical warfare agent digestion material specifically comprises the following steps:
[0047] (1) zirconium oxychloride octahydrate (ZrOCl 2 ·8H 2 O, 4.3943g, 13.6mmol) and ferric chloride hexahydrate (FeCl 3 ·6H 2 O, 0.3686g, 1.36mmol) was dissolved in 150mL water, stirred at a speed of 100r / min for 20min to obtain 4+ and Fe 3+ mixed solution;
[0048] (2) adding the strong ammonia water containing Zr dropwise with a mass concentration of 25% 4+ and Fe 3+ in the mixed solution to pH 9.0 to obtain ZrFe(OH) x precipitation;
[0049] (3) ZrFe(OH) x Rinse the precipitate with deionized water for 5 times, centrifuge to dry, dry at room temperature, and grind it into a powder with a particle size of x .
Embodiment 2
[0051] The preparation method of chemical warfare agent digestion material specifically comprises the following steps:
[0052] (1) zirconium oxychloride octahydrate (ZrOCl 2 ·8H 2 O, 3.4527g, 10.72mmol) and ferric chloride hexahydrate (FeCl 3 ·6H 2 O, 1.1584g, 4.29mmol) was dissolved in 150mL water, stirred at a speed of 100r / min for 20min to obtain 4+ and Fe 3+ mixed solution;
[0053] (2) adding the strong ammonia water containing Zr dropwise with a mass concentration of 25% 4+ and Fe 3+ in the mixed solution to pH 9.0 to obtain ZrFe(OH) x precipitation;
[0054] (3) ZrFe(OH) x Rinse the precipitate with deionized water for 5 times, centrifuge to dry, dry at room temperature, and grind it into a powder with a particle size of x .
Embodiment 3
[0056] The preparation method of chemical warfare agent digestion material specifically comprises the following steps:
[0057] (1) zirconium oxychloride octahydrate (ZrOCl 2 ·8H 2 O, 2.84g, 8.82mmol) and ferric chloride hexahydrate (FeCl 3 ·6H 2 O, 1.667g, 6.17mmol) was dissolved in 150mL water, stirred at a speed of 100r / min for 20min to obtain 4+ and Fe 3+ mixed solution;
[0058] (2) adding the strong ammonia water containing Zr dropwise to the concentration of 28% 4+ and Fe 3+ in the mixed solution to pH 9.0 to obtain ZrFe(OH) x precipitation;
[0059] (3) ZrFe(OH) x Rinse the precipitate with deionized water for 5 times, centrifuge to dry, dry at room temperature, and grind it into a powder with a particle size of x .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com