Synthesis method of alkyl (meth) acrylate
A technique for the synthesis of alkyl acrylates, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as reducing the yield of target products and increasing the risk of polymerization tower blockage, and achieves maintenance Low cost, good catalytic effect, and reduced addition by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
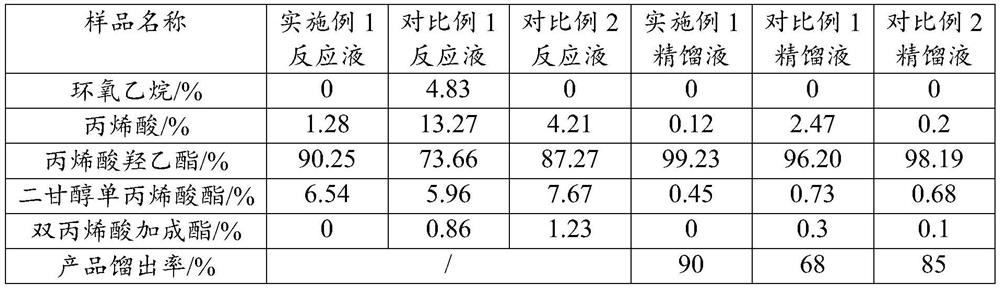
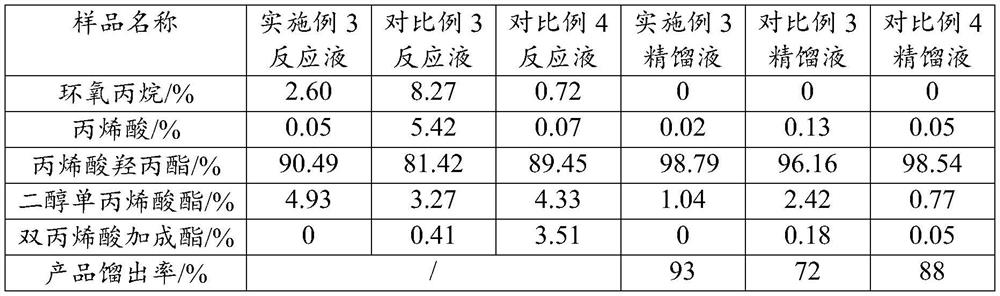
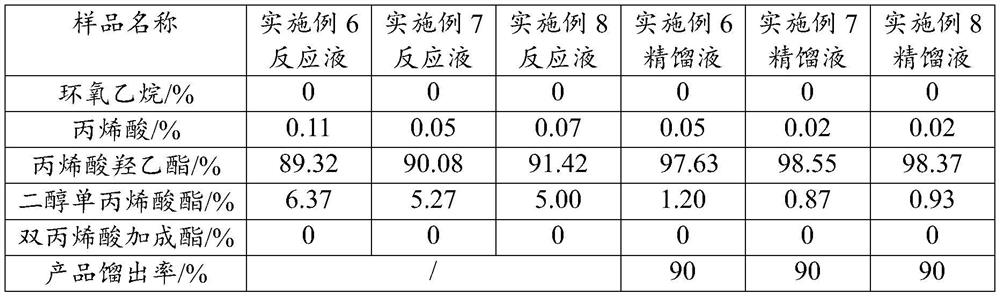
[0025] A kind of synthetic method of alkyl (meth)acrylate, it specifically comprises the following steps: specifically comprises the steps:
[0026] S1, put the heterogeneous solid acid catalyst, polymerization inhibitor and (meth)acrylic acid into the reactor;
[0027] S2. After raising the temperature to 50-90°C, feed epoxy into the reactor at a flow rate of 1200-1600kg / h for reaction, and the temperature during the reaction should not exceed 90°C;
[0028] S3. After the addition of epoxy oxide is completed, heat and mature at 50°C-90°C for 1-3h, filter the reaction solution to remove the non-homogeneous solid acid catalyst, keep the pressure below 50mTorr, and control the temperature at 70°C-90°C Rectification, that is, the target product.
[0029] Wherein, the heterogeneous solid acid catalyst is composed of a carrier and iron ions and chromium ions loaded on the carrier; the carrier is a layered silicate with a pillar structure, preferably, the layered silicate is select...
Embodiment 1
[0033] 1. Preparation of heterogeneous solid acid catalyst
[0034] Dissolve 4.5kg of chromium acetate and 1kg of ferric chloride in an appropriate amount of deionized water (the molar ratio of chromium ion to iron ion is 3:1), impregnate 16.6kg of montmorillonite and 13.4kg of saponite (average CEC is about 90mmol / 100g) in Sonicate the above solution for 4 hours at 80°C, filter and wash with water until there is no floating color, dry and grind it into powder; place the above powder in the air and calcinate at 550°C for 3 hours to prepare a heterogeneous solid acid catalyst for future use.
[0035] Two, the preparation of hydroxyethyl acrylate
[0036] Specifically include the following steps:
[0037] S1. Feeding and nitrogen placement: Vacuum pump 2500kg of acrylic acid into the reactor, put in 8.4kg of phenothiazine, and 21kg of the prepared heterogeneous solid acid catalyst, and replace the internal air with nitrogen, and the nitrogen pressure is ≥0.02 during replacement...
Embodiment 2
[0062] One, the preparation method of heterogeneous solid acid catalyst is identical with embodiment 1.
[0063] Two, the preparation of hydroxyethyl methacrylate
[0064] Specifically include the following steps:
[0065] S1. Feeding and nitrogen placement: Vacuum pump 3010kg of methacrylic acid into the reactor, put in 8.4kg of phenothiazine, 21kg of the above-mentioned heterogeneous solid acid catalyst, and replace the internal air with nitrogen, and the nitrogen pressure is ≥0.02MPa during replacement , the vacuum degree is pumped to -0.02MPa.
[0066] S2. Response: After the nitrogen setting is completed, close the vacuum valve. Raise the temperature to 50°C, feed 1700kg of ethylene oxide, and slowly raise the temperature to 65-70°C, control the pressure to ≤0.25MPa, and feed the ethylene oxide at a rate of 1200-1600kg / h (subject to the actual pressure).
[0067] S3. Ripening: After the feeding of ethylene oxide is completed, close the discharge valve of the ethylene o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com