Hesperetin quinoline hydrazone derivative with anti-tumor activity as well as preparation method and application thereof
A technology of hesperetin and quinoline hydrazone is applied to hesperetin quinoline hydrazone derivatives, preparation methods and application fields thereof, and achieves the effects of improving antitumor activity, broadening action targets, and broadening action mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
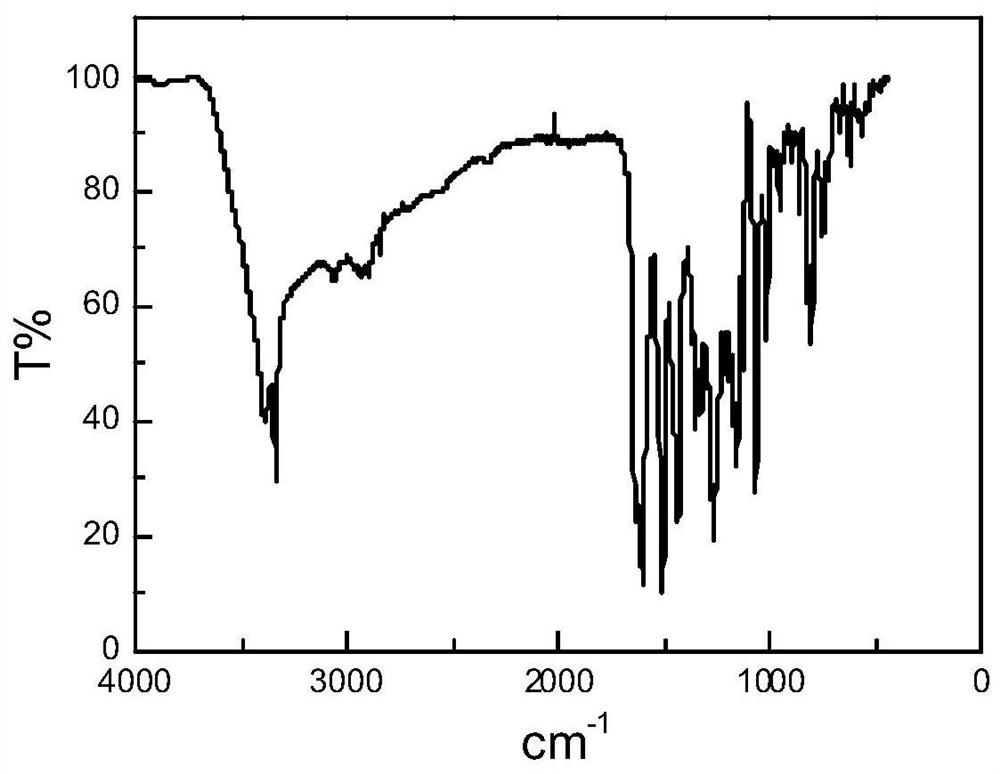
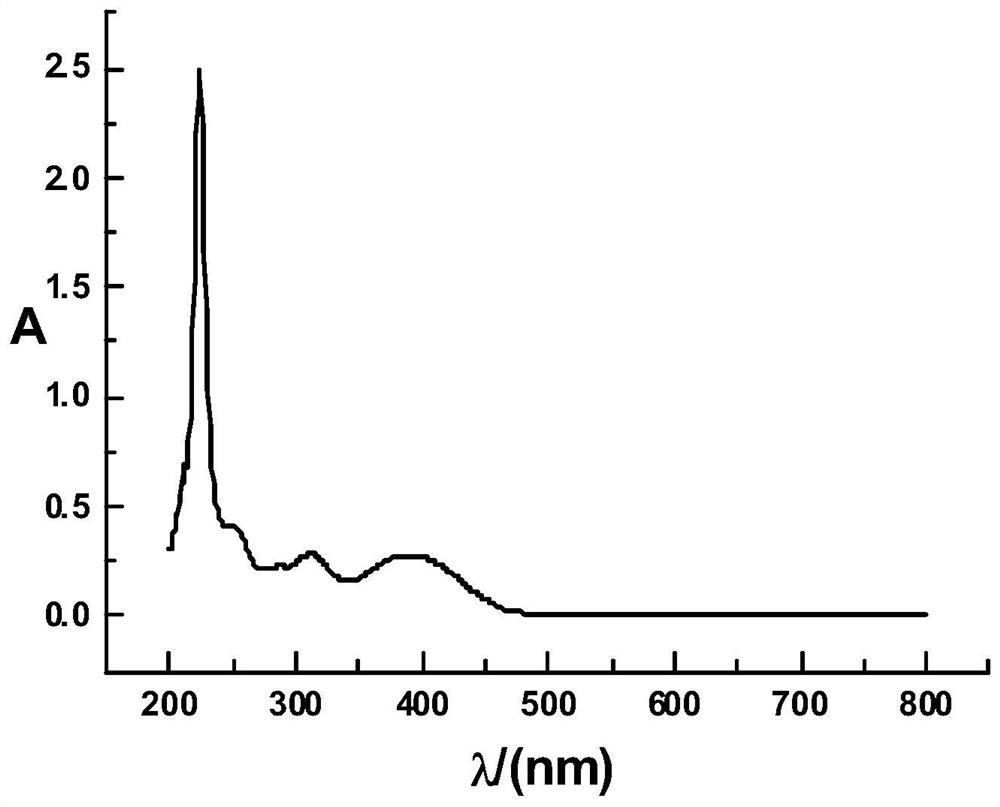
[0033] Hesperetin-4-quinoline hydrazone, the molecular formula is C 25 h 21 N 3 o 5 , the molecular structure formula is:
[0034]
[0035] The preparation method of hesperetin-4-quinoline hydrazone comprises the following steps:
[0036] (1) Dissolve 20 mmol 2-chloroquinoline in absolute ethanol, add 8 mL of hydrazine hydrate (in excess) with a mass fraction of 80%, and react at reflux temperature for 8 hours to obtain a crude intermediate;
[0037] (2) The intermediate crude product is recrystallized with absolute ethanol to obtain an orange flaky crystal 2-hydrazinoquinoline intermediate;
[0038] (3) Dissolve 12mmol of 2-hydrazinoquinoline intermediate and 10mmol of hesperetin in absolute ethanol respectively, mix them, add 2 to 5 drops of glacial acetic acid to catalyze, react at reflux temperature, and monitor the reaction progress with TLC, and proceed with the reaction. Precipitate orange precipitate;
[0039] (4) After the reaction is finished, suction filter...
experiment example
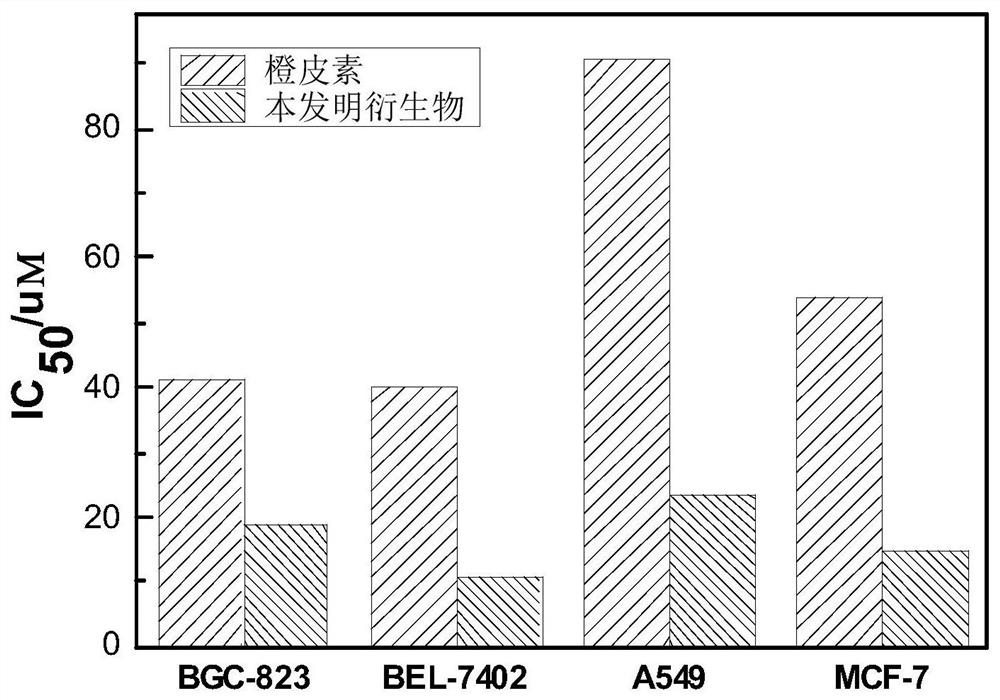
[0044] The present invention is used for in vitro antitumor activity experiment, and assay method (MTT method) is as follows:
[0045] 1. Cell culture
[0046] The human gastric cancer cell BGC-823, human liver cancer cell BEL-7402, human lung cancer cell A549 and human breast cancer cell MCF-7 selected for this experiment need to be treated with 1% (v / v) double antibiotics (100 U / mL chain Mycin and 100 U / mL penicillin), 89% (v / v) 1640 basal medium and 10% (v / v) fetal bovine serum complete medium. at 37°C with 5% CO 2Cultured in an incubator, the medium was changed on the second day, and passaged on the third day. The experimental study took the cells that grew stably in the logarithmic phase.
[0047] 2. Cell passage
[0048] Observe the density of the cells. When the cells proliferate to about 80%, discard the original culture medium, add sterile PBS solution to wash 1-2 times, add 0.25% trypsin, digest for 5 minutes, add complete medium, blow the cells gently, Collect t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com