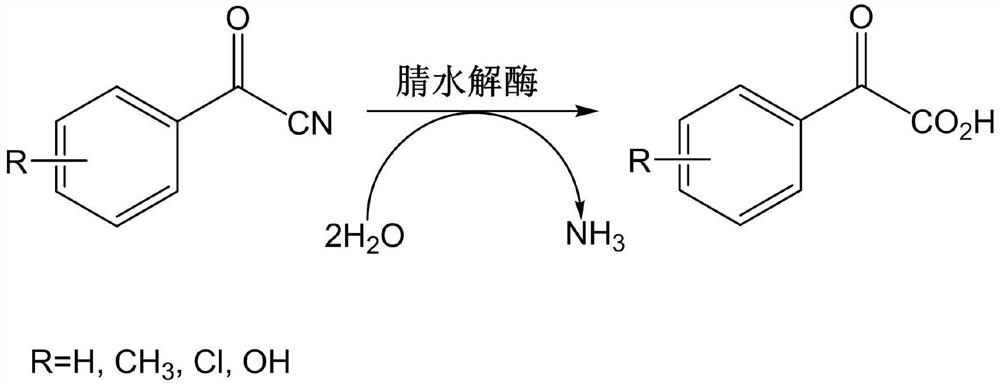
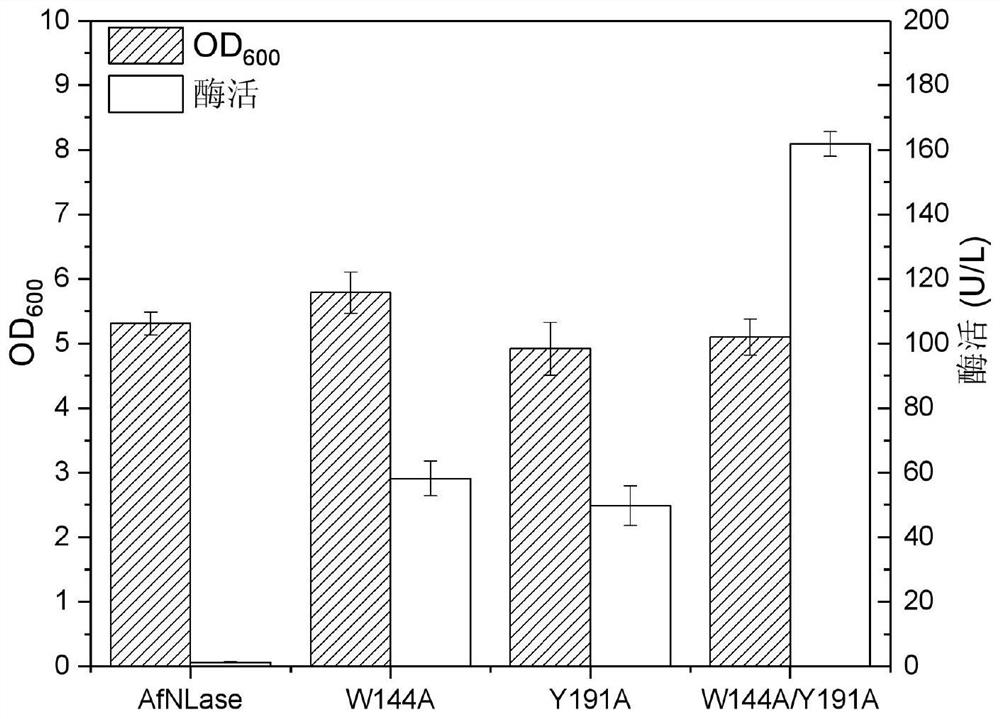
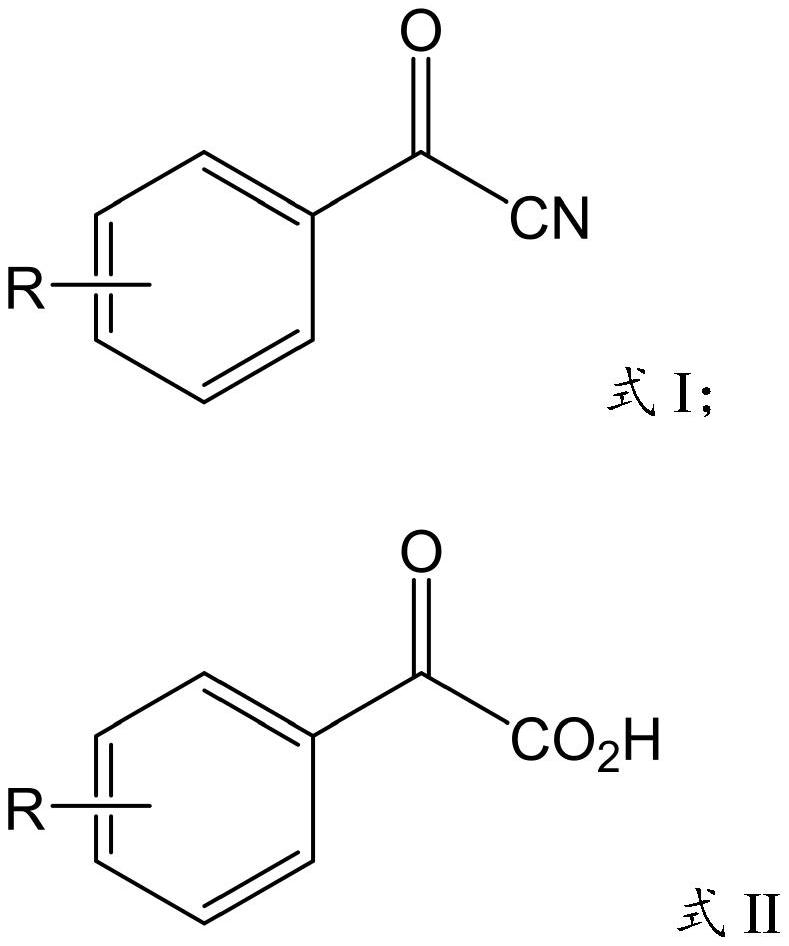
Nitrilase mutant and application thereof in preparation of benzoylformic acid compounds
A kind of nitrilase, mutant technology, applied in biological field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Construction of wild-type nitrilase recombinant expression strain
[0021] Entrusted Beijing Qingke Xinye Biotechnology Co., Ltd. to provide codon optimization and gene synthesis services, derived from the wild-type nitrilase (AfNLase) gene of Alcaligenes faecalis (Alcaligenes faecalis), the gene sequence is shown in SEQ ID NO.1, and the amino acid sequence is shown in Shown in SEQ ID NO.2. This gene was cloned between the restriction sites EcoRI and HindIII on the pET-28a(+) plasmid to obtain the pET-28a(+)-AfNLase recombinant plasmid. The recombinant plasmid pET-28a(+)-AfNLase was transfected into the host Escherichia coli E.coli BL21(DE3) to obtain the recombinant genetic engineering bacteria E.coli BL21(DE3)-pET-28a(+)-AfNLase.
[0022] LB medium was used to activate and cultivate the genetically engineered bacteria E.coli BL21(DE3)-pET-28a(+)-AfNLase. The specific formula of LB medium is: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized wat...
Embodiment 2
[0044] 1. The cultivation of engineered bacteria
[0045] Inoculate the engineered bacteria containing related genes into 5 mL LB liquid medium containing 50 μg / mL kanamycin, and culture with shaking at 37°C for 12 hours. Transfer to 500 mL of fresh LB liquid medium also containing 50 μg / mL Kan, shake culture at 37 °C until OD600 reaches about 0.8, add IPTG to its concentration of 0.5 mM, and induce culture at 28 °C for 16-18 h. After the cultivation, the culture solution was centrifuged at 4000rpm for 10min, the supernatant was discarded, the bacterial cells were collected, and stored in a -70°C ultra-low temperature refrigerator until use.
[0046] 2. Determination of Enzyme Activity
[0047] The thalli cells collected after the culture were finished were washed twice with 0.25M PB buffer (pH 7.5). Afterwards, the bacteria were resuspended in 0.25M PB buffer (pH 7.5) twice the volume of the fermentation broth, and the cells were disrupted by ultrasonic to obtain a crude en...
Embodiment 3
[0051] Example 3 Genetically engineered bacteria catalyze benzoyl nitrile to generate acetophenone
[0052] Take the fermentation broth of the genetically engineered bacteria E.coli BL21(DE3)-pET-28a(+)-AfNLase-W144A constructed in Example 1, centrifuge at 4000rpm for 10min to collect the thalline, weigh 0.5g of the wet thalline and wash it with 200mL 0.25M Resuspend broken cells in phosphate buffer (pH 7.5) to obtain crude enzyme solution and add it to a 250mL round bottom flask; weigh 2.0g of benzoyl nitrile into the flask, turn on magnetic stirring, and control the reaction temperature in a water bath to 40°C for hydrolysis For the reaction, 4M NaOH was used to control the reaction pH=7.5. After reacting for 8 hours, the contents of benzoylnitrile and acetophenone in the reaction system were detected by liquid chromatography. The substrate conversion rate was 93%, and the concentration of acetophenone acid was 10.6 g / L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pre-denatured | aaaaa | aaaaa |
| Extend | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com