Leukocyte binding C reactive protein rapid detection card and preparation method thereof
A technology of reactive protein and detection card, which is applied in the field of protein immunological detection, can solve the problems of elevated white blood cell count and inability to completely rule out infection, etc., and achieve the effect of simple detection operation, fast and accurate quantitative detection results, and improved accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] In the present embodiment, the preparation solution involved includes:
[0067] Reagent card hydrophilic treatment solution: 10mM PBS buffer + 1% TW20;
[0068] Gold-labeled antibody complex solution: 10mM borate buffer + 6% sucrose + 2% BSA;
[0069] Glass fiber membrane pretreatment solution: 50Mm borate buffer + 0.3% TW20 + 2% BSA;
[0070] Antibody coating solution: 10mM PBS buffer + 3% methanol;
[0071] pH regulator: 1% potassium carbonate solution;
[0072] Blocking solution: 10% BSA aqueous solution.
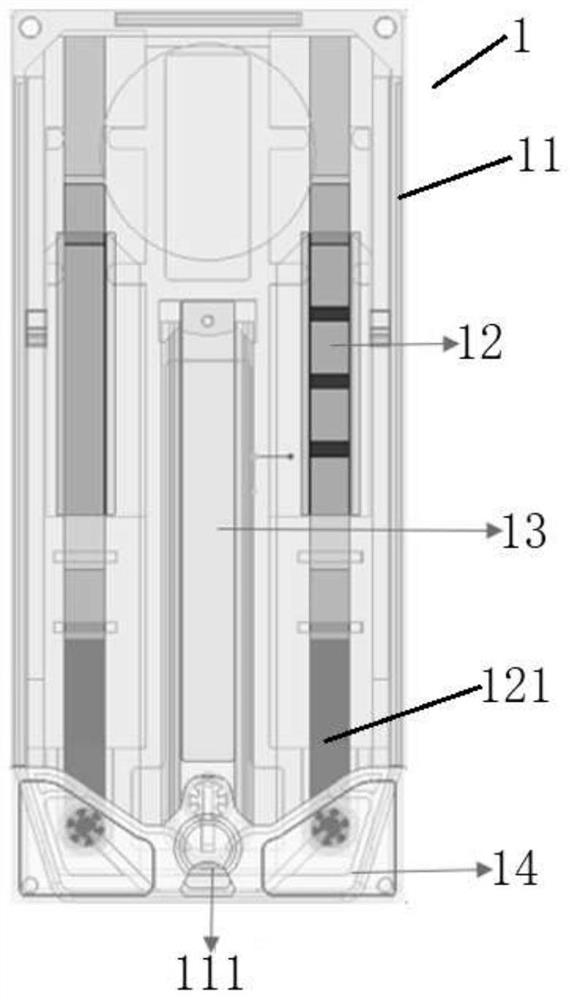
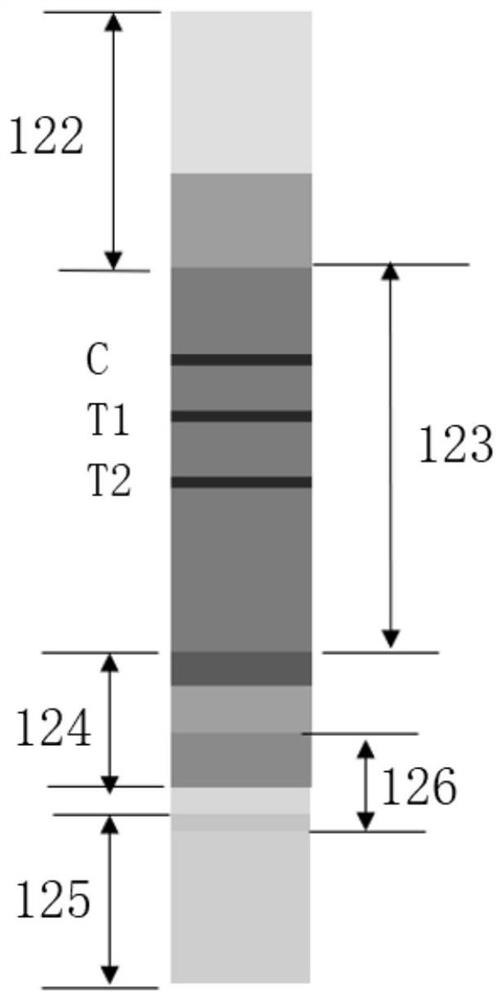
[0073] The leukocyte-binding C-reactive protein rapid detection component of the present invention utilizes blood cell staining and counting and C-reactive protein immunochromatographic reaction principles to simultaneously realize rapid quantitative detection of leukocytes and C-reactive protein in whole blood samples. The leukocyte-binding C-reactive protein rapid detection component includes a detection card 1 and a sample diluent, the sample diluent is con...
Embodiment 2
[0086] Embodiment 2 performance detection test
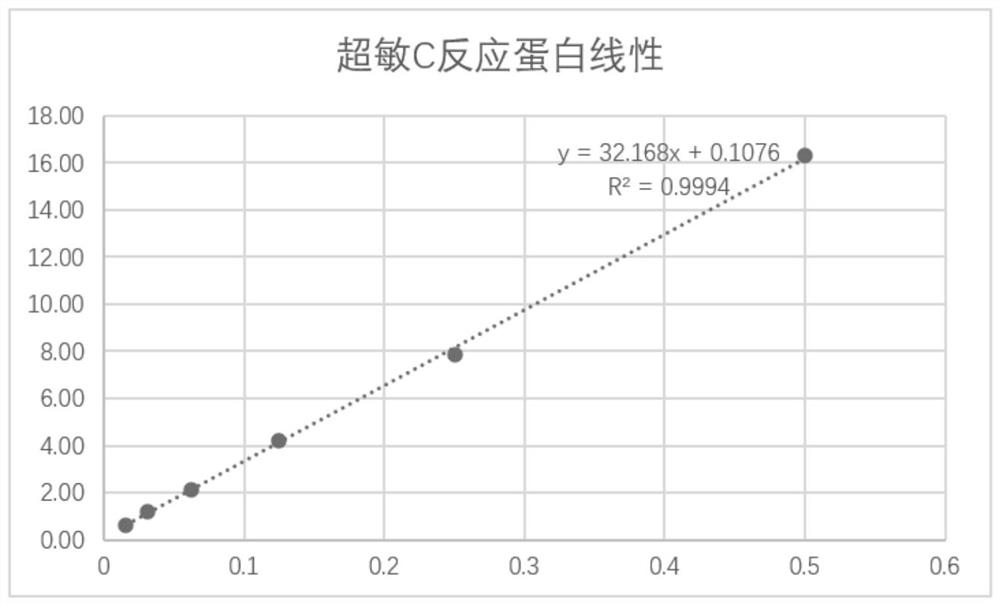
[0087] In this example, the detection limit, analytical specificity, accuracy, hook effect, precision, and linear range of the leukocyte-binding C-reactive protein rapid test card were respectively tested to evaluate whether they meet the requirements of the experimental design. .
[0088]In this embodiment, the standard products used for performance evaluation are human hypersensitive C-reactive protein No. 8C72 of Millipore Company and CELL-DYN 29Plus Control of Abbott Company for blood cell analysis. All experiments require the experimenters to be familiar with the detection method and instrument operation; use appropriate standards; the reagents used in the experiment should be within the validity period.
[0089] 1. Detection limit (this performance evaluation is for the C-reactive protein detection reagent in the kit)
[0090] The sample treatment solution in the kit in Example 1 was used as the blank solution, and the b...
Embodiment 3
[0160] Embodiment 3 Clinical Serum Sample Detection
[0161] Experimenters should be familiar with the detection method and instrument operation; the reagents used in the experiment should be of the same batch number and within the validity period. The whole blood used in the experiment is freshly collected, and has been clinically tested to determine the assigned value.
[0162] Randomly select 0.5mg / L9 / L9 / L whole blood samples of 30 cases, 12mg / L>C-reactive protein content>3mg / L, 18×10 9 / L>Total leukocytes>10mg / ml×10 9 70 cases of / L whole blood samples were added dropwise to the test card in Example 1 for blind test and the statistical results are shown in Table 16-17 below.
[0163] Table 16 C-reactive protein test results
[0164]
[0165]
[0166]
[0167] Table 17 Total white blood cell test results
[0168]
[0169]
[0170] It can be seen that the clinical accuracy of the detection card of the present invention is CV<15%, which meets the needs of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com