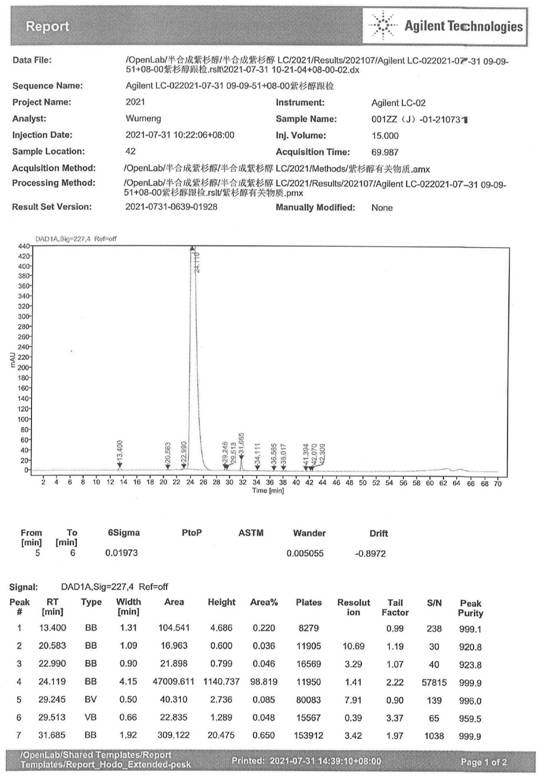
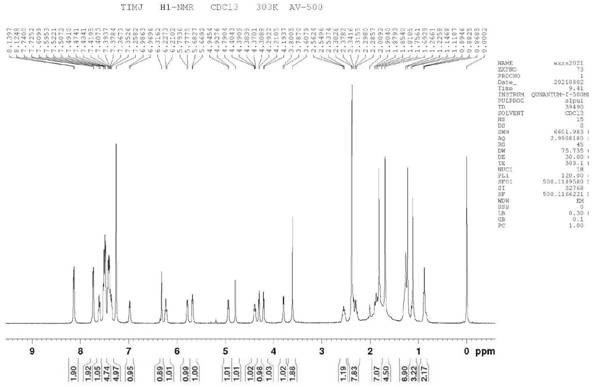
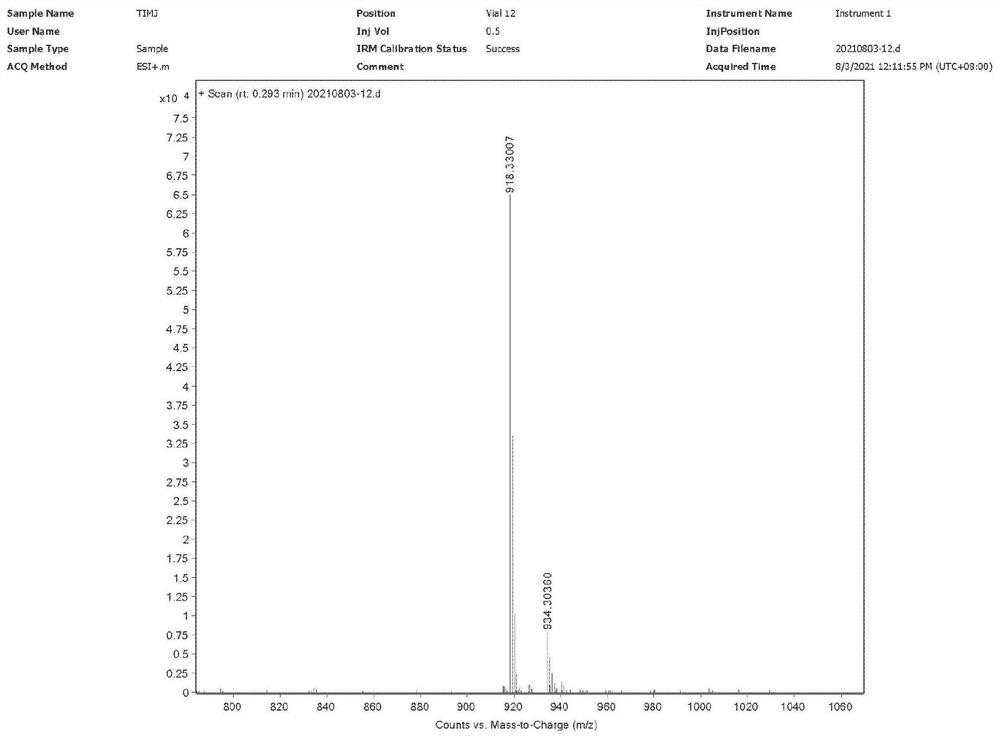
Preparation method of 10-acetyl paclitaxel
A technology of paclitaxel and 10-DAB, which is applied in the field of preparation of 10-acetylpaclitaxel, and achieves the effects of low cost, being beneficial to large-scale industrial production and easy to judge.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] S1: 10g precursor 10-deacylated baccatin III (10-DAB), followed by 10-DAB instead, 10-DAB was dissolved in 130g dichloromethane and 49g pyridine, and 12g chloroformic acid-2,2,2 was added dropwise -Trichloroethyl ester, stirred and reacted under ice bath (0°C) for 2h, after the reaction was completed, quenched the reaction with water, washed with hydrochloric acid and salt water respectively, collected and concentrated the organic phase, added 15g of toluene to make a slurry, and dried by suction to obtain 15.1g of IMJ- 1;
[0038] S2: Dissolve 15.1g IMJ-1 in 150g toluene, add 6.0g paclitaxel side chain acid and 0.91g 4-dimethylaminopyridine, add dropwise 6.0g N,N'-dicyclohexylcarbodiimide (with a small amount of dilute with toluene), stir the reaction at room temperature around 20°C for 2h, after the reaction is over, add water to quench the reaction, filter with suction, add ethyl acetate to the filtrate, then extract with saline, concentrate the organic phase, dissol...
Embodiment 2
[0048] S1: Dissolve 10g of 10-DAB in 180g of dichloromethane and 50g of pyridine, add 12g of 2,2,2-trichloroethyl chloroformate dropwise, stir at room temperature (around 5°C) for 1 hour, add water to quench the reaction after completion of the reaction , washed with hydrochloric acid and salt water respectively, collected and concentrated the organic phase, added 15g of toluene to make a slurry, and dried by suction to obtain 14.8g of IMJ-1;
[0049] S2: Dissolve 14.8g IMJ-1 in 135g toluene, add 7.4g paclitaxel side chain acid and 0.90g 4-dimethylaminopyridine, add dropwise 7.4g N,N'-dicyclohexylcarbodiimide (with a small amount of dilute with toluene), stir the reaction at room temperature around 23°C for 4h, after the reaction is over, add water to quench the reaction, filter with suction, add ethyl acetate to the filtrate, then extract with saline, concentrate the organic phase, dissolve it in 30ml ethyl acetate, add dropwise 60ml Recrystallized from n-heptane, filtered an...
Embodiment 3
[0055] S1: Dissolve 100g of 10-DAB in 2600g of dichloromethane and 500g of pyridine, add 125g of 2,2,2-trichloroethyl chloroformate dropwise, stir and react at 0-10°C for 0.5h, add water to quench the reaction after completion of the reaction, Wash with hydrochloric acid and brine respectively, collect and concentrate the organic phase, add 150 g of toluene to make a slurry, and filter and dry to obtain 149.1 g of IMJ-1;
[0056] S2: Dissolve 148g IMJ-1 in 1480g toluene, add 68g paclitaxel side chain acid and 11.8g 4-dimethylaminopyridine, add dropwise 74g N,N'-dicyclohexylcarbodiimide (diluted with a small amount of toluene) , Stir the reaction at about 23°C for 3.5h. After the reaction is over, add water to quench the reaction, filter with suction, add ethyl acetate to the filtrate, then extract with saline, concentrate the organic phase, dissolve it in 300ml ethyl acetate, add dropwise 600ml n-heptane Recrystallized, filtered and dried to obtain 187g IMJ-2;
[0057] S3: Di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com