3, 6-dinitramine triazolo triazole, ionic salt thereof, preparation method and application
A technology of triazolo and dinitroamine, which is applied in the field of new high-energy energetic compounds to achieve the effects of reducing friction sensitivity, enhancing π-π interaction, and improving thermal stability and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention provides a specific embodiment of the preparation method of 3,6-dinitroamine[1,2,4]triazolo[4,3-b][1,2,4]triazole, comprising the following steps: Dissolve 3,6-diamino[1,2,4]triazolo[4,3-b][1,2,4]triazole in sulfuric acid, add nitric acid dropwise, and react for 2-4 hours, and the reaction solution Quenched by adding to crushed ice, filtered with suction to obtain a yellow solid, dried to obtain 3,6-dinitroamine[1,2,4]triazolo[4,3-b][1,2,4]triazole .
[0039] In a specific application, the system temperature is kept below 0° C. during the whole preparation process.
[0040] In the present invention, the sulfuric acid is concentrated sulfuric acid with a mass fraction of 98% or oleum with a mass fraction of 20%, and the nitric acid is fuming nitric acid or anhydrous nitric acid.
[0041] In the present invention, when the above-mentioned types of sulfuric acid and nitric acid are used, the volume ratio of sulfuric acid to nitric acid is 3:1˜4:1. ...
Embodiment 1 2
[0056] The synthesis of embodiment 1 dinitramine triazolotriazole (1)
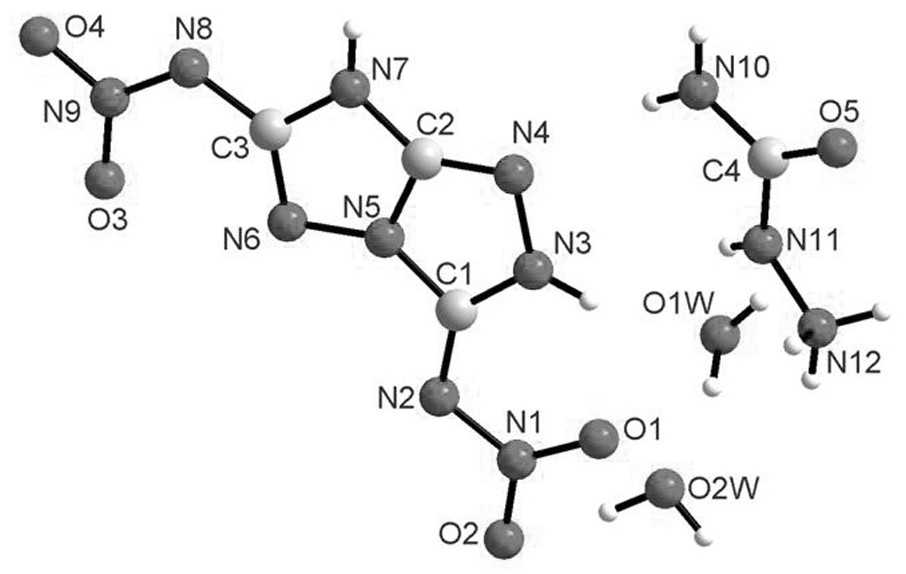
[0057] Add 1.39g of 3,6-diamino[1,2,4]triazolo[4,3-b][1,2,4]triazole (10mmol) that was ground and pulverized in advance to 15g of oleum, and wait After ultrasonic dissolution is complete, then keep the temperature not exceeding -5°C, and slowly add 4mL of fuming nitric acid. Reacted for 3.5h, keeping the temperature at -5°C. It was then quenched with 40 g of crushed ice and filtered to obtain a yellow solid that was dissolved in CaCl 2 After drying in medium overnight, the product was obtained (yield 78%).
[0058] In the present invention, the 3,6-diamino[1,2,4]triazolo[4,3-b][1,2,4]triazole is self-made in the laboratory, and the specific preparation method can be found in C. Bian, Q. Lei, J. Zhang, et al, Polyhedron, 2021, 201, 115158.
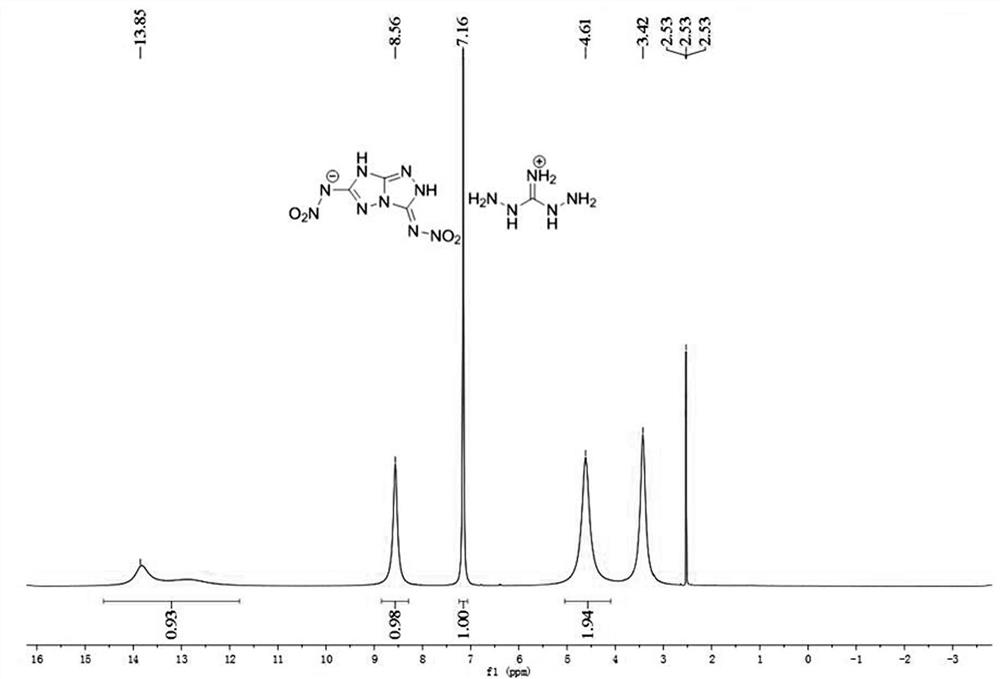
[0059] Product structure identification:
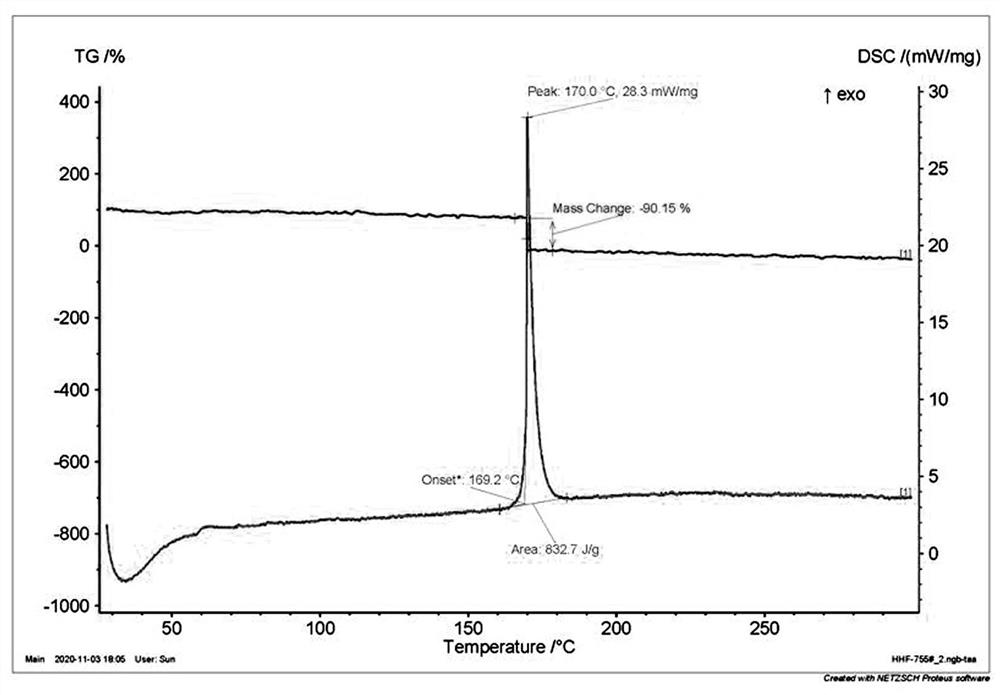
[0060] Decomposition temperature: 121°C (DSC, the melting point and decomposition temperature mentioned below are peak t...
Embodiment 2 2
[0066] The synthesis of embodiment 2 dinitramine triazolotriazoles (1)
[0067] Add 1.98g of 3,6-diamino[1,2,4]triazolo[4,3-b][1,2,4]triazole (14.2mmol) that was pulverized in advance to 17.1g of concentrated sulfuric acid, After the ultrasonic solution is completely dissolved, then keep the temperature not exceeding -2°C, slowly add 3.8mL of anhydrous nitric acid, react for 2.5h, and keep the temperature at -5°C. Then quenched with 56g of crushed ice, filtered and compacted to obtain a yellow solid, which was dissolved in CaCl 2 Dry overnight in medium, then wash with appropriate amount of acetonitrile, filter, and put the obtained filter cake into an oven for 2 hours at 50° C. to vacuum-dry to obtain 2.73 g of dinitramine triazolotriazole (yield: 84%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| impact sensitivity | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com