Preparation method and application of photocuring temperature-induced phase change ionic gel electrolyte
An ion gel, light curing technology, applied in the direction of solid electrolyte, non-aqueous electrolyte, hybrid capacitor electrolyte, etc., can solve the problems of single function, difficult sealing, environmental pollution, etc., to avoid environmental pollution, avoid air bubbles, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] In order to solve the above technical problems, the first aspect of the present invention provides a method for preparing a photocurable temperature-induced phase change ion gel electrolyte, comprising the following steps:
[0040] (1) Synthesis of polymer monomer: mix polymer and diisocyanate, add catalyst, heat to 70-90°C in nitrogen atmosphere for 1-2.5h, then drop to 40-60°C and add acrylic acid monomer, react After 1-2 hours, the reaction is terminated, and the polymer monomer is obtained;
[0041] (2) Preparation of photocurable temperature-induced phase change ionic gel electrolyte: mix polymer monomer with bistrifluoromethanesulfonimide salt, add photoinitiator, mix well, inject into mold, and irradiate with ultraviolet light for 30s ~10min to prepare photocurable temperature-induced phase change ion gel electrolyte.
[0042] In one embodiment, the molar ratio of the polymer to the diisocyanate is 1:(1-2).
[0043] In one embodiment, the molar ratio of the pol...
Embodiment 1
[0075]Synthesis of Polymer Monomer-1: Mix PPG-4000 (CAS No.: 25322-69-4) with a molecular weight of 4000 and isophorone diisocyanate (CAS No.: 4098-71-9), add dilauric acid Dibutyltin (CAS No.: 77-58-7) was heated to 80°C for 1.5 hours in a nitrogen atmosphere and then lowered to 50°C. Add hydroxyethyl methacrylate (CAS No.: 868-77-9) and react for 1 hour to terminate Reaction to obtain polymer monomer-1; the molar ratio of PPG-4000 to isophorone diisocyanate is 1:1.3; the molar ratio of PPG-4000 to hydroxyethyl methacrylate is 1:1.3; dilauric acid The weight of dibutyltin is 1% of the total weight of PPG-4000 and isophorone diisocyanate;
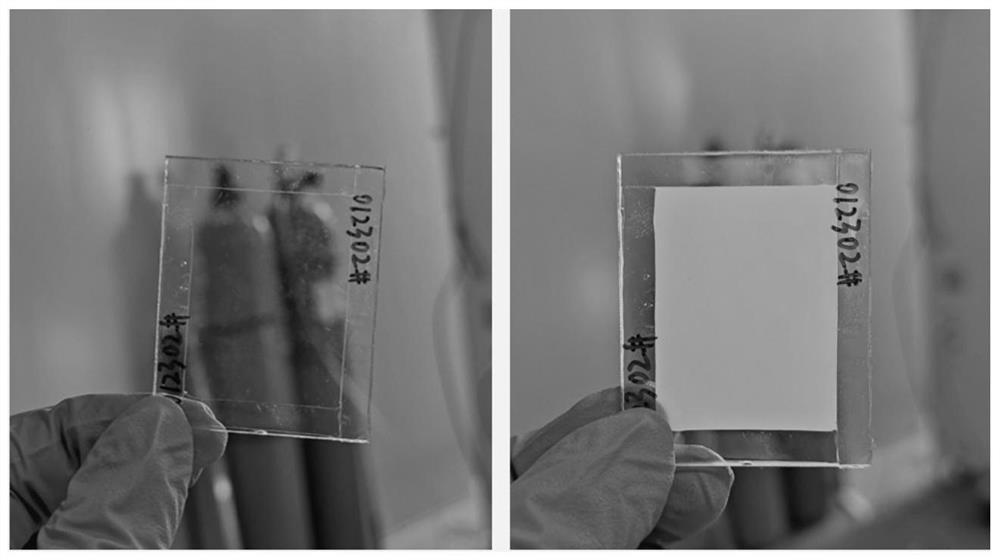
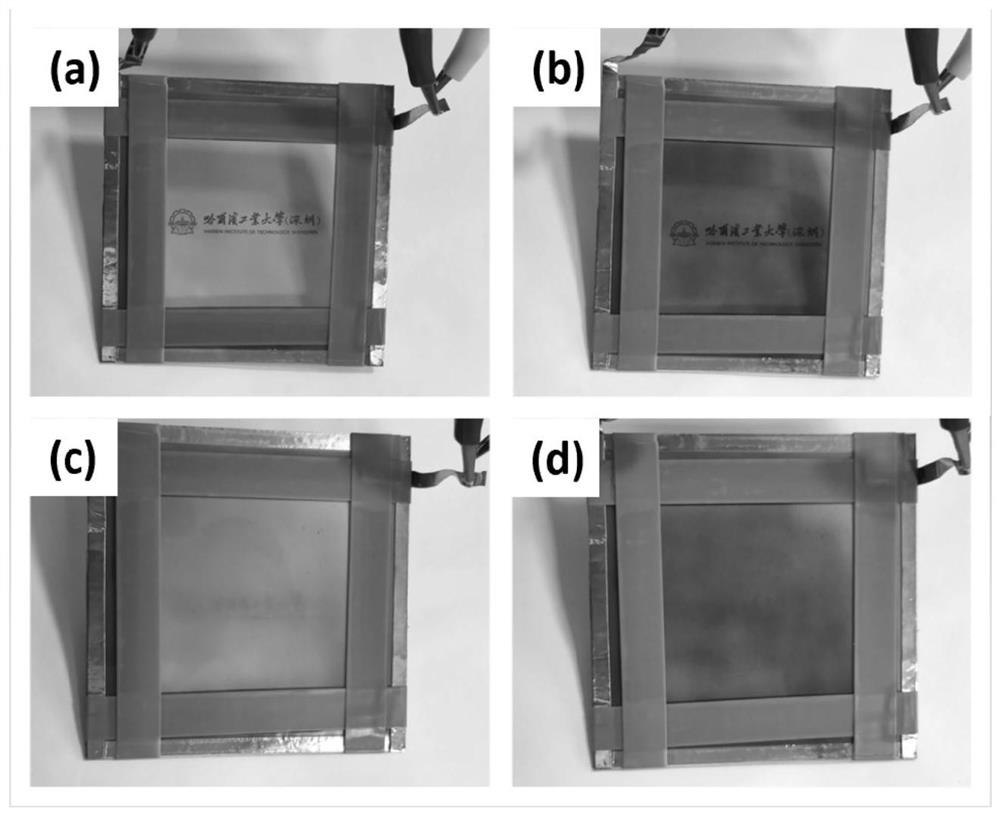
[0076] Preparation of photocurable temperature-induced phase change ion gel electrolyte-1: polymer monomer-1 and 1-ethyl-3-methylimidazole bistrifluoromethanesulfonimide salt (CAS number: 174899-82- 2) After mixing, add 2-hydroxy-2-methylpropiophenone (CAS No.: 7473-98-5) and mix well, then pour into the double-layer transparent glass, irr...
Embodiment 2
[0080] Synthesis of Polymer Monomer-2: Mix PPG-4000 with a molecular weight of 4000 and isophorone diisocyanate, add dibutyltin dilaurate, heat to 80°C for 1.5h in a nitrogen atmosphere, then drop to 50°C and add After reacting hydroxyethyl methacrylate for 1 hour, the reaction was terminated, and the polymer monomer-2 was obtained; the molar ratio of PPG-4000 and isophorone diisocyanate was 1:1.8; the molar ratio of PPG-4000 and hydroxyethyl methacrylate The molar ratio is 1:1.8; the weight of dibutyltin laurate is 1% of the total weight of PPG-4000 and isophorone diisocyanate;
[0081] Preparation of photocurable temperature-induced phase change ionic gel electrolyte-2: mix polymer monomer-2 with 1-ethyl-3-methylimidazole bistrifluoromethanesulfonimide salt and add 2-hydroxyl-2 - Methylpropiophenone is mixed evenly and poured into double-layer transparent glass, and after being irradiated by ultraviolet light for 5 minutes, the double-layer transparent glass is disassembled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com