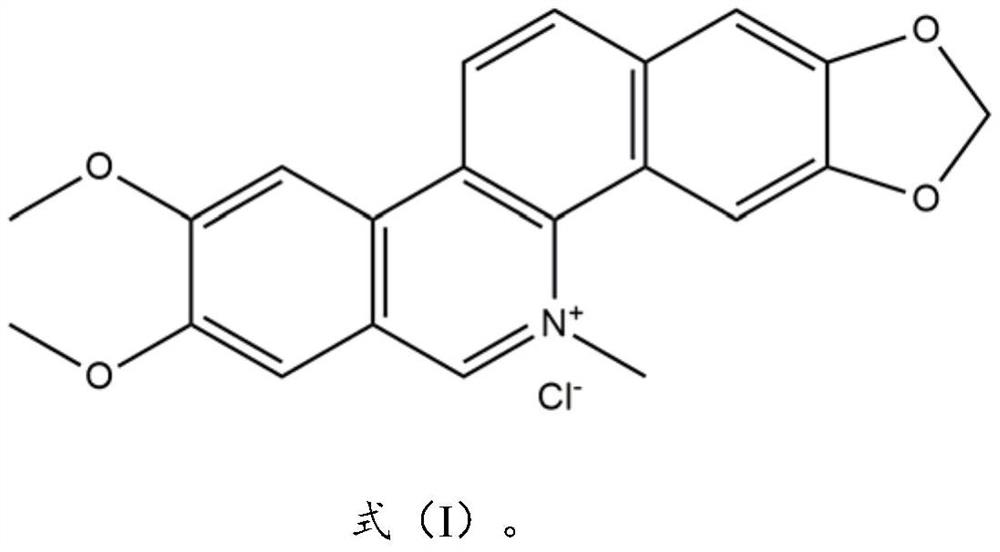
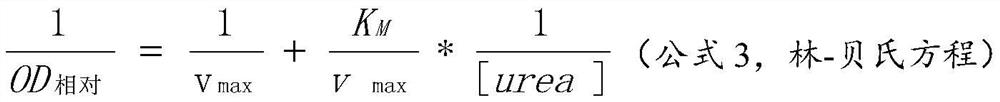Application of nitidine chloride in preparation of urease inhibitor
A technology of chlorinated acanthine and urease inhibitors, which is applied in the field of natural compound applications, can solve the problems of acetohydroxamic acid losing its effect, and achieve the effects of enriching optional types, reducing drug resistance, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Research on the inhibitory effect of chlorinated diacid on urease
[0038] Mix the canavalia urease solution and a series of concentration test drug solutions, and incubate at 37°C for 20 minutes; add urea solution, and react in the dark for 20 minutes at room temperature; add Berthelot color developing solution in the dark for 10 minutes, Pipette 200 μL of the incubation solution onto a 96-well plate, and measure the absolute value of OD at 595 nm on a microplate reader. Each concentration was done 3 times in parallel. The blank sample was replaced by the solvent of each dilution, and the other operations were the same as above, and the OD blank was measured. Calculate the corresponding OD relative value according to formula 1. According to formula 2, the residual enzyme activity (Residual activity, RA) was obtained, and the corresponding half inhibitory concentration IC was obtained by GraphPad 50 .
[0039] OD 相对 =OD 绝对 –OD 空白 (Formula 1)
[0040]...
Embodiment 2
[0042] Example 2: Research on the type of inhibition of urease by chlorinated double-sided alkaloids
[0043] A certain concentration of cana urease was mixed with a series of concentrations of echinacea chloride solution (0, 25, 50, 100 μM), and incubated at 37° C. for 20 minutes. Then add a series of concentrations of urea solution (0.9375-30mM) at room temperature to react in the dark for 20 minutes, then develop color according to the method of Example 1 and measure the OD 绝对 value and obtain the corresponding OD 相对 value. The solvent of the added substance was used as a blank control, and the parallel determination was performed 3 times. The experiment passes the reciprocal of the reaction speed (1 / V, that is, 1 / OD 相对 ) to the reciprocal (1 / [urea]) of the substrate concentration to make a Lineweaver-Burk diagram, and finally obtain the kinetic parameter K by combining the L-B curve with formula 3 M , v max , and through the L-B curve to obtain the inhibition constant...
Embodiment 3
[0046] Example 3: Kinetic study on the inhibition of urease by chlorinated dihedral
[0047] Take the same volume of a series of concentrations of Echinacea chloride solution (0, 25, 50, 100 μM) and a certain concentration of canavalia urease and mix well, then incubate in a 37°C incubator for 20min, then add an equal volume of urea at room temperature The solution was reacted in the dark for 20 minutes (incubation system) or directly added to an equal volume of urea solution without incubation in an incubator and reacted in the dark for 20 minutes (incubation system). Then pipette the reaction solution into a 1.5mL centrifuge tube at different time intervals (0-30min), then develop color according to the method of Example 1 and measure the OD 绝对 value and obtain the corresponding OD 相对 value. The solvent of the added substance was used as a blank control, and the parallel determination was performed 3 times.
[0048] The OD obtained from the above two reaction systems 相对 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com