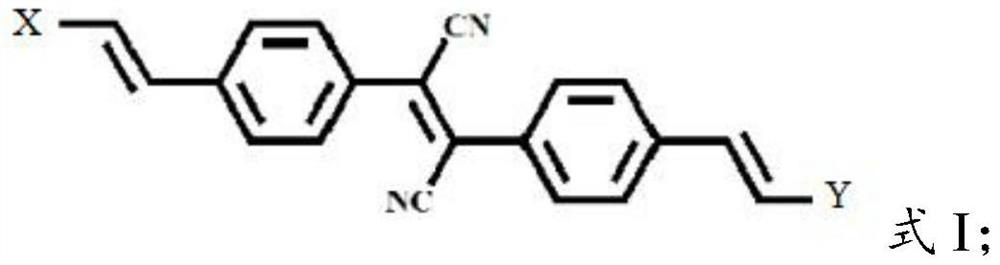
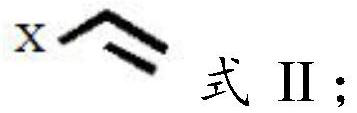Cyano styrene derivative, preparation method and application thereof, polymer detection probe and fluorescence detection method
A technology of cyanostyrene and derivatives, which is applied in fluorescence/phosphorescence, measurement devices, photovoltaic power generation, etc., to achieve the effect of improving the depth of fluorescence imaging and large two-photon absorption cross-sectional area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The present invention provides the preparation method of cyanostyrene derivative described in above-mentioned technical scheme, comprises the following steps:
[0061] P-bromophenylacetonitrile, iodine, sodium methylate solution and the first solvent are mixed to carry out bimolecular oxidative coupling reaction to obtain double bromo intermediates;
[0062] The bisbromine intermediate product, electron-donating group compound, Pd(OAc) 2 、Ag 2 CO 3 Mix with the second solvent, carry out Heck reaction, obtain cyanostyrene derivative.
[0063] The monad-containing compound has a structure shown in formula II:
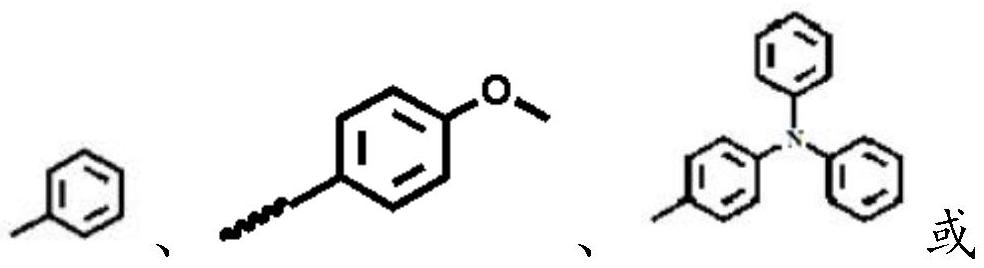
[0064] Formula II; In formula II, X is
[0065] In the present invention, unless otherwise specified, the required preparation materials are commercially available products well known to those skilled in the art.
[0066] The present invention mixes p-bromophenylacetonitrile, iodine, sodium methoxide solution and a first solvent to carry out a bimolecula...
Embodiment 1
[0095] Dissolve p-bromophenylacetonitrile (5.00g, 25.5mmol) and iodine (6.57g, 25.5mmol) in dry ether (100mL), dissolve sodium methoxide (2.89g, 5.3mmol) in methanol solution (8.68g, 14.61 mL), mixed the obtained ether mixture with sodium methoxide solution, stirred in a dry ice bath for 0.5 h, raised the temperature to 0°C, replaced the dry ice bath with ice water, and stirred for 4 h. % of hydrochloric acid to quench the reaction; after stirring the resulting mixed material for 12 hours, the resulting material was filtered, and the solid separated from the resulting product was washed with methanol at 0°C and then rinsed with water, and then the resulting mixture was dried to obtain a bis-bromo intermediate ;
[0096] The electron-donating group compound 4-dianilino-styrene (0.52nmol), the bisbromo intermediate (0.52mmol), Pd(OAc) 2 (0.026mmol) and Ag 2 CO 3 (0.31mmol) was placed in a 50mL Schlenk test tube, added in 15mL toluene, stirred and reacted at 110°C for 18h, and...
Embodiment 2
[0099] Prepare according to the method for embodiment 1 and obtain two bromine intermediates;
[0100] The electron-donating group compound 4-methoxystyrene (1.04nmol), the bisbromo intermediate (0.52mmol), Pd(OAc) 2 (0.052mmol) and Ag 2 CO 3 (0.62mmol) was placed in a 50mL Schlenk test tube, added in 15mL toluene, stirred and reacted at 110°C for 18h, the resulting product system was purified on a silica gel column, and the reagents used were dichloromethane and petroleum ether (volume ratio: 1:1), Obtain cyanostyrene derivatives, the structural formula is:
[0101] Recorded as DOB.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com