Preparation method of 7-fluoro-2-oxoindoline-4-carboxylic acid
An indoline and oxo technology, applied in the direction of organic chemistry, etc., can solve the problems of large-scale production danger, hazardous waste liquid environment, and high risk coefficient, and achieves easy industrialization amplification, improved safety, and high reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
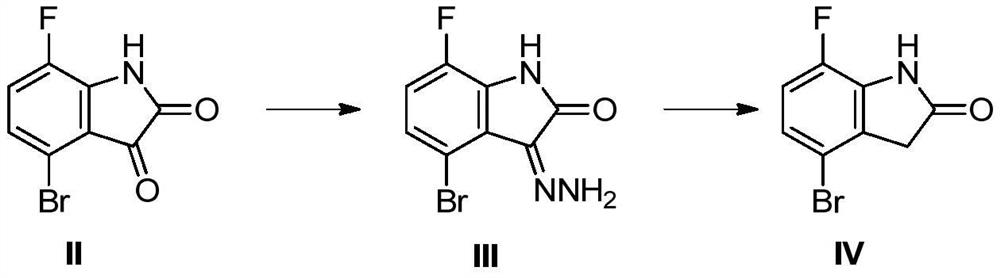
[0048] Add 250ml of ethylene glycol into a 500ml three-necked flask, add 50g (205mmol) of the compound shown in formula (II) and 15.4g (250mmol) of hydrazine hydrate (80%), stir for 30min, control the internal temperature at 55-65°C, and react 2 -6h, the central control raw material disappeared, and the reaction was completed. Cool down to room temperature and filter to obtain 60 g of the crude product represented by formula (III) (the next reaction can be directly carried out without further treatment).
Embodiment 2
[0050] Add 500ml of ethylene glycol into a 500ml three-necked flask, add 60g of the crude product shown in (III) obtained above, add 1.7g (20.5mmol) of sodium acetate, and raise the reaction temperature to 135-145°C, react for 8-16h, and control The reaction is complete. Concentrate under reduced pressure to 1 / 3 of the original volume, cool down to 0-10°C, filter, rinse with 100ml of water, and vacuum-dry to obtain 40g of the compound represented by formula (IV), with a purity of ≥98%.
Embodiment 3
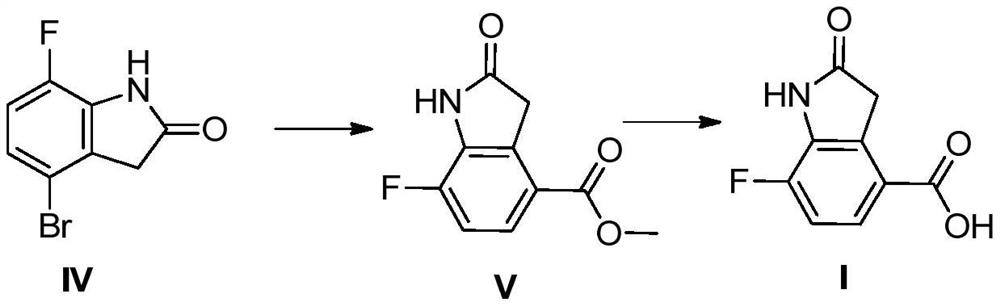
[0052] In the pressure reactor of 500ml, add methanol 300ml, add the compound 30g shown in (IV) obtained above, add triethylamine 27ml, (dppf)PdCl 2 1.55g, replace with CO three times, control the pressure of CO in the reactor to 0.5MPa; control the reaction temperature at 115-125°C, react for 10h, cool down to room temperature, concentrate the solvent, add DMF and DCM, stir for 30min, and filter to obtain formula (V) The compound shown is 23g, with a purity≥98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com