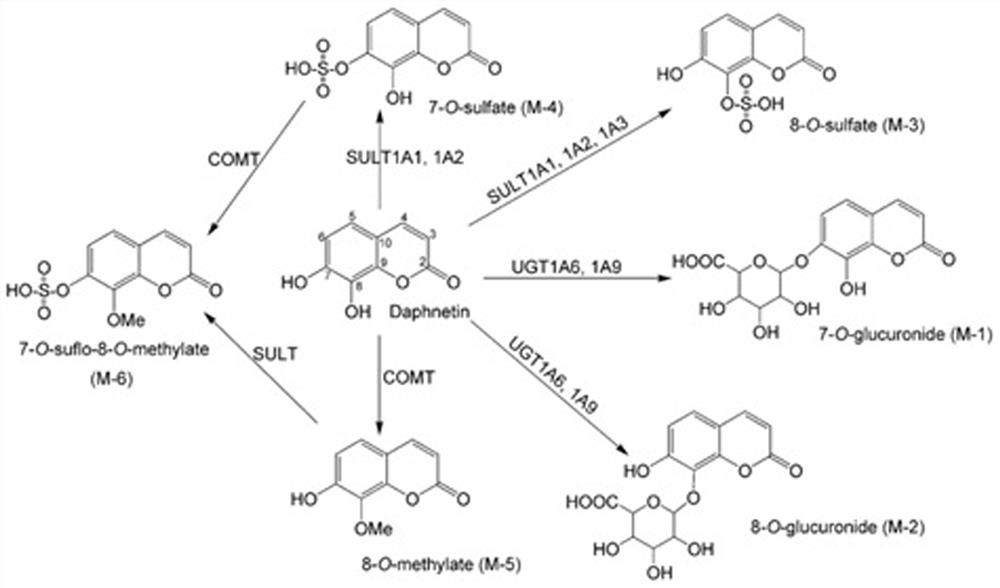
Overall level characterization method of COMT enzyme activity and application thereof
An enzyme activity and level technology, applied in biochemical equipment and methods, microbial measurement/inspection, material inspection products, etc., can solve problems such as difficult COMT enzyme activity evaluation, undetermined methyl products, complex metabolic pathways, etc., to achieve Good specificity, suitable for widespread application, good detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The present embodiment determines to use 3-aminocoumarin as IS, and the specific operations are as follows:
[0040] High signal intensity of the target analyte is necessary for an MS-based analysis method. The chromatographic separation conditions and mass spectrometry parameters were optimized.
[0041] Based on the analytes (daphnetin and daphnetin-Me 100ng / mL) of Q1 full-scan mass spectrometry, it was found that the strong signal of daphnetin-Me in positive ion mode was significantly higher than that in negative ion mode, while that of daphnetin in positive and negative ion modes The comparison of the MS signals of the above results shows that the positive ion mode is beneficial for the determination of daphnetin and 8-methyl metabolites, such as figure 2 shown. Based on this observation, 3-aminocoumarin was finally selected as the proton-accepting ability of 3-aminocoumarin, as well as its similarity with daphnetin and the 8-methyl metabolite in terms of structu...
Embodiment 2
[0043] This embodiment provides the hydrolysis treatment process of daphnetin metabolites, the specific operations are as follows:
[0044] (1) Select SD rats (male, 200±20g), and place the rats in a standard environment with a temperature of 25±2° C. and a humidity of 50±5% for 2 weeks (12 / 12h dark / light cycle) before the experiment. Fast overnight before administration;
[0045] (2) Dissolve daphnetin with Tween-80, pour it into rats, and collect rat urine for a period of time;
[0046] (3) Take the same amount of urine, add sulfotransferase and uridine diphosphate glucuronyltransferase to hydrolyze it;
[0047] (4) Daphnetin and its 8-methyl metabolites remained in the final urine.
Embodiment 3
[0049] The present embodiment provides the analytical conditions of liquid chromatography and liquid phase mass spectrometry detection system, specifically as follows:
[0050] The detection system includes liquid chromatography and liquid chromatography mass spectrometry. The analysis conditions are as follows: All analyzes are carried out by LC-MS / MS system, which includes LC-30AD×2 infusion pump, DGU-20A5 online degasser, SIL-30AC automatic sampling device, CTO-20A column furnace, CBM- 20A system controller and 8045 triple quadrupole mass spectrometer. The computer is equipped with LC-MS / MS solution software (V version 5.85). Acchrom Xcharge C18 column (2.1×150mm, 5μm) was used for chromatographic separation, and the column temperature was 40°C. The mobile phase composition was a mixture of 0.1% formic acid-acetonitrile (63:37, v / v). The flow rate was 0.3 mL / min, and the temperature of the autosampler was maintained at 10°C. The injection volume was 10 μL, and the run t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com