Electrochemical synthesis method of 2-aryl-1, 2-diacetophenone
A technology of benzophenone and synthesis method, which is applied in the direction of electrolytic components, electrolytic process, electrolytic organic production, etc., can solve the problems of large pollution, high energy consumption, complex ligand metal catalysts, etc., and achieve mild reaction conditions and cheap raw materials Easy to obtain and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
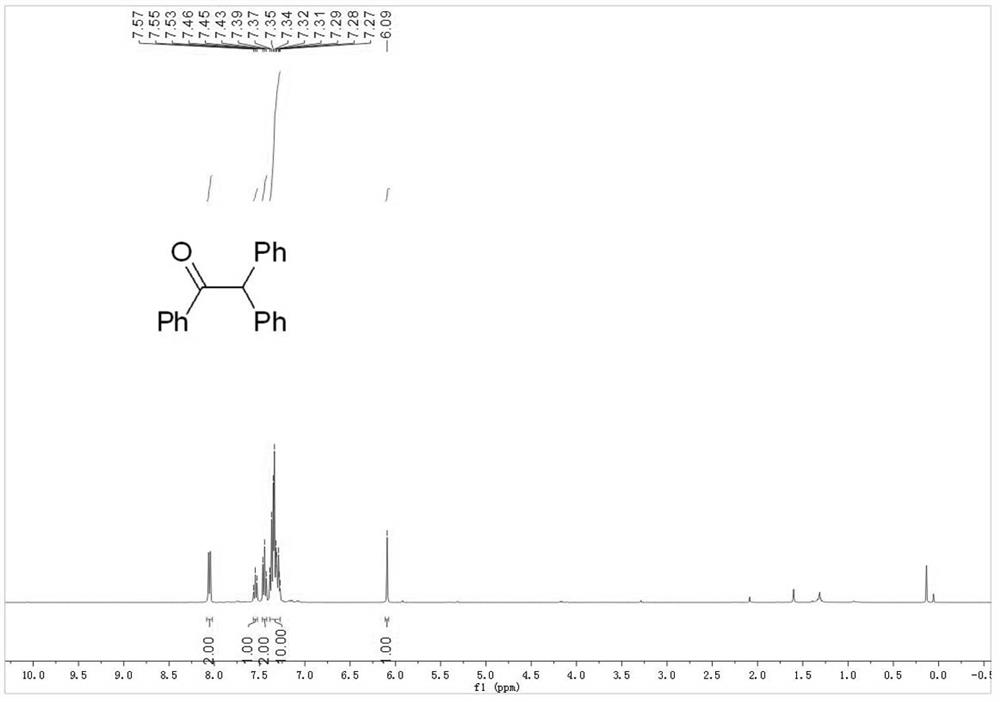
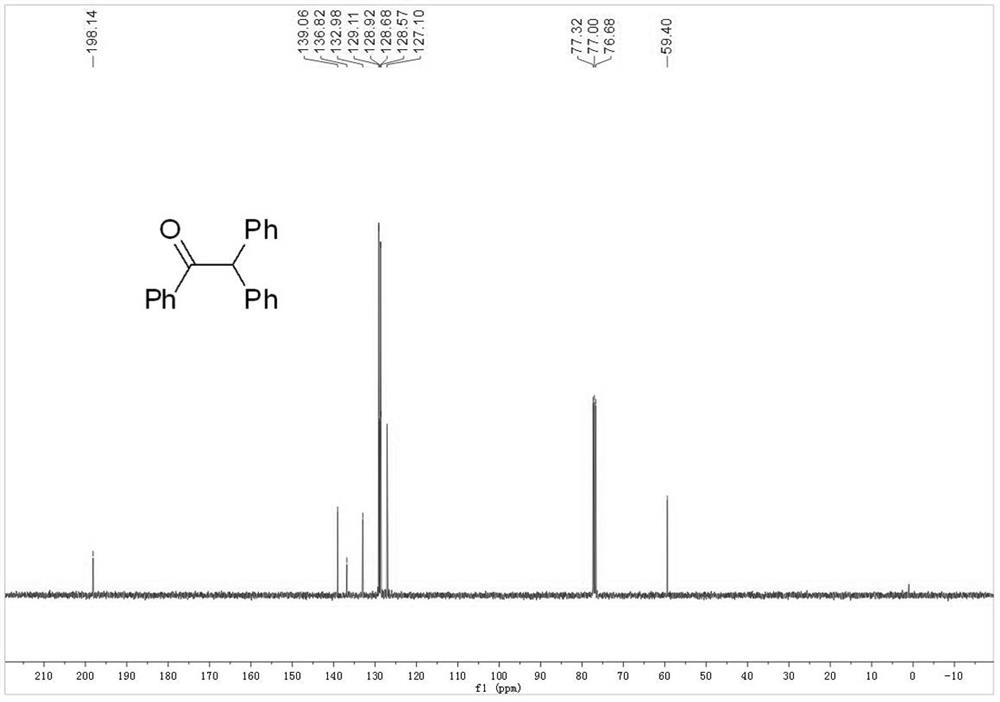
Embodiment 1
[0040] The reaction equation is:
[0041]
[0042] Wash and dry a 10ml chicken mouth bottle, prepare a platinum wire and a silver wire to pass through the rubber stopper of a bottle as a wire, and connect two pieces of graphite felt (2cm×1cm×0.5cm) to the lower end of the wire , use Teflon film to separate the two pieces of graphite felt, and then use Teflon thread to fasten the two pieces of graphite felt. The graphite felt connected with platinum wire is used as the anode of the electrolysis reaction, and the graphite felt connected with the silver wire is used as the cathode of the electrolysis reaction. Add 0.2mmol triarylethylene (compound of formula 1a) and 0.1mmolLiClO 4 , the reaction bottle was filled with oxygen 3 times, and then 5 mL of dry CH was added 3 CN. The reaction was carried out at a constant voltage of 5V at room temperature, and the reaction was completed after 1.5h. After the reaction, the solvent was removed under reduced pressure, and then separ...
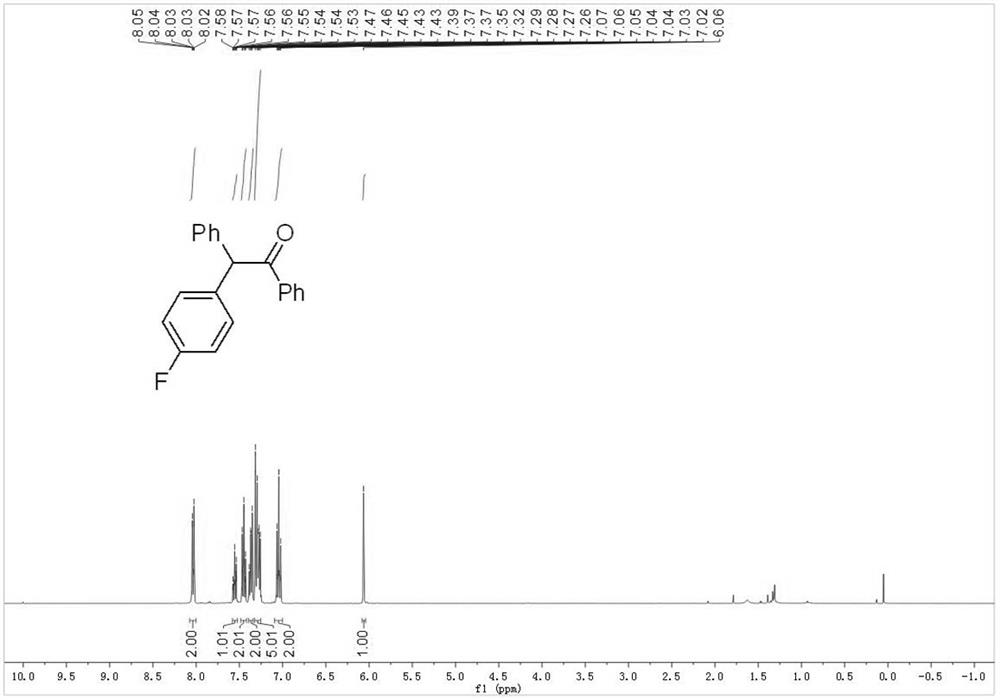
Embodiment 2
[0047] The reaction equation is:
[0048]
[0049] Using the compound of formula 1b as the raw material, other operations were the same as in Example 1 to obtain 49 mg of the compound of formula 2b with a yield of 84%. The nuclear magnetic data of gained formula 2b compound is:
[0050] 1 H NMR (400MHz, Chloroform-d) δ=8.08–8.00(m,2H),7.58–7.52(m,1H),7.49–7.42(m,2H),7.39–7.34(m,2H),7.33–7.25 (m,5H),7.09–7.00(m,2H),6.06(s,1H).
[0051] 13 C NMR (100MHz, Chloroform-d) δ=198.03, 161.93 (d, J=245.9Hz), 138.9, 136.66, 134.90 (d, J=3.3Hz), 133.12, 130.70 (d, J=8.0Hz), 128.96 ,128.90,128.85,128.63,127.27,115.49(d,J=21.4Hz),58.51.
[0052] 19 F NMR (376 MHz, Chloroform-d) δ = -115.59.
Embodiment 3
[0054] The reaction equation is:
[0055]
[0056] Using the compound of formula 1c as the raw material, other operations were the same as in Example 1 to obtain 50 mg of the compound of formula 2c with a yield of 82%. The nuclear magnetic data of gained formula 2c compound is:
[0057] 1 H NMR (400MHz, Chloroform-d) δ=8.00–7.98(m,2H),7.55–7.51(m,1H),7.45–7.39(m,2H),7.36–7.25(m,7H),7.23–7.18 (m,2H),6.01(s,1H).
[0058] 13 C NMR (100 MHz, Chloroform-d) δ = 197.79, 138.61, 137.68, 136.60, 133.19, 133.11, 130.50, 128.98, 128.92, 128.90, 128.79, 128.67, 127.36, 58.68.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com