Preparation and application of gel electrolyte for anti-freezing zinc-based battery
A gel electrolyte and battery technology, applied in the direction of electrolyte immobilization/gelation, secondary batteries, circuits, etc., can solve the problem of low ionic conductivity of gel electrolyte, harsh preparation conditions, flexible zinc-based batteries cannot resist low temperature, etc. problems, to achieve stable electrochemical performance, excellent electrochemical performance, and lower condensation temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
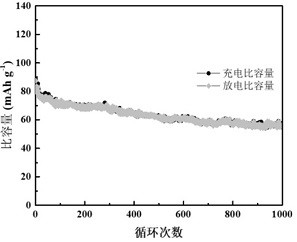
[0026] At 30°C, 3 g of sodium alginate was dissolved in 40 mL of deionized water. Add acrylamide monomer and zwitterionic monomer with a total mass of 10g in turn and stir evenly, then add potassium persulfate and N, N'-methylenebisacrylamide, so that potassium persulfate in the mixture is 0.05% mol of the monomer , N, N'-methylenebisacrylamide is 0.01% mol of the monomer, mixed thoroughly and evenly, poured into a polytetrafluoroethylene mold with a thickness of 1mm, and reacted at 60°C for 3h to form a hydrogel by free radical polymerization. Dissolve 30g of zinc chloride and 10g of lithium chloride in 50mL of deionized water to prepare a water electrolyte. Under the condition of 30° C., the assembled hydrogel was immersed in the above aqueous electrolyte solution for 3 hours to finally obtain a gel electrolyte.
Embodiment 2
[0028] At 40°C, 2.5g of sodium alginate was dissolved in 40mL of deionized water. Add acrylamide monomer and zwitterionic monomer with a total mass of 15g in turn and stir evenly, then add potassium persulfate and N, N'-methylenebisacrylamide, so that potassium persulfate in the mixture is 0.1% mol of the monomer , N, N'-methylenebisacrylamide is 0.02% mol of the monomer, mixed thoroughly and evenly, poured into a polytetrafluoroethylene mold with a thickness of 1mm, and reacted at 60°C for 4h to form a hydrogel by free radical polymerization. Dissolve 30g of zinc chloride and 10g of lithium chloride in 50mL of deionized water to prepare a water electrolyte. Under the condition of 40° C., the polymerized hydrogel was immersed in the above water electrolyte for 4 hours to finally obtain a gel electrolyte.
Embodiment 3
[0030] At 50°C, 2 g of sodium alginate was dissolved in 40 mL of deionized water. Add acrylamide monomer and zwitterionic monomer with a total mass of 25g in turn and stir evenly, then add potassium persulfate and N, N'-methylenebisacrylamide, so that potassium persulfate in the mixture is 0.15% mol of the monomer , N, N'-methylenebisacrylamide is 0.03% mol of the monomer, mixed thoroughly and evenly, poured into a polytetrafluoroethylene mold with a thickness of 1mm, and reacted at 60°C for 5h to form a hydrogel by free radical polymerization. Dissolve 30g of zinc sulfate and 10g of lithium chloride in 50mL of deionized water to prepare a water electrolyte. Under the condition of 30° C., the polymerized hydrogel was immersed in the above water electrolyte for 12 hours to finally obtain a gel electrolyte.
PUM
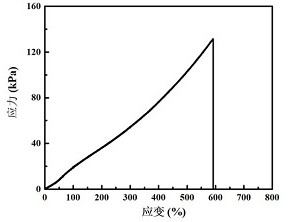
| Property | Measurement | Unit |
|---|---|---|
| Maximum tensile stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com