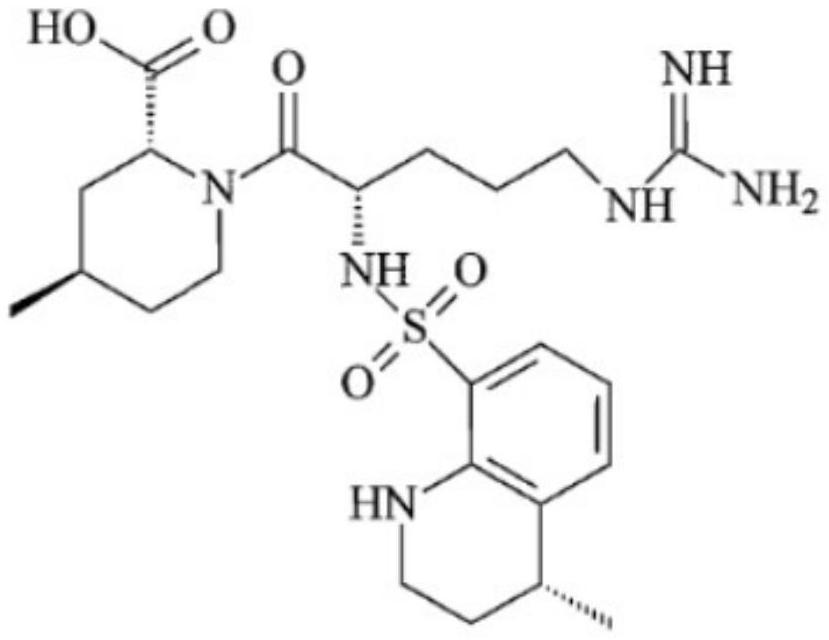
Argatroban injection liposome and preparation method thereof
A lipid and plastid technology of argatroban, which is applied in the field of argatroban injection liposome and its preparation, can solve problems such as not being suitable for large-scale production, affecting the clinical application of drugs, and complicated preparation methods, and achieving The effect of reducing toxicity, improving encapsulation efficiency, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
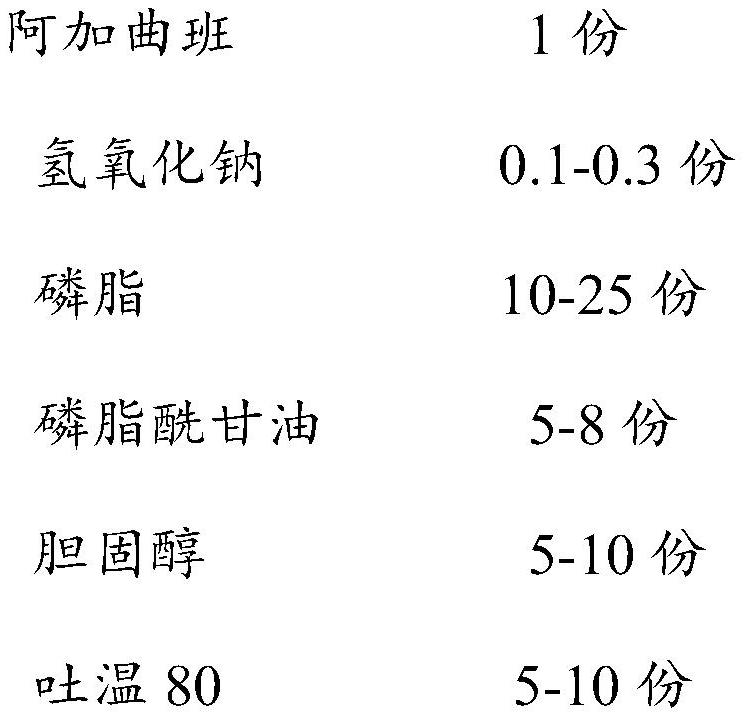
[0033] Embodiment 1: the preparation of argatroban liposome injection, specifically according to the following prescription by weight
[0034]
[0035] Argatroban liposome injection was prepared by the following preparation process:
[0036] 1) Dissolve the prescribed amount of cultured phosphatidylethanolamine, phosphatidylglycerol, cholesterol and Tween 80 in 120ml of sodium chloride solution, stir and sonicate for 15 minutes to obtain blank liposomes;
[0037] 2) steam-sterilize the blank lipid obtained in step 1) and sonicate for 15 minutes;
[0038] 3) Mix and dissolve the prescribed amount of argatroban and sodium hydroxide in 300ml of water for injection, heat the sterilized blank liposomes to 56°C and keep warm, slowly add the aqueous solution of argatroban under stirring, continue Incubate at 56° C. for 10-15 minutes, add 120 ml of a citrate buffer solution with a pH of 6.5, and mix well to obtain argatroban liposome injection.
Embodiment 2
[0039] Embodiment 2: the preparation of argatroban liposome injection, specifically according to the following prescription by weight
[0040]
[0041] Argatroban liposome injection was prepared by the following preparation process:
[0042] 1) Dissolve the prescribed amount of dicetyl phospholipid, phosphatidylglycerol, cholesterol and Tween 80 in 150ml of ethanol solution, heat and stir, spin evaporate to remove ethanol, and ultrasonicate the remaining components for 15 minutes to obtain blank liposomes;
[0043] 2) steam-sterilize the blank lipid obtained in step 1) and sonicate for 15 minutes;
[0044] 3) Mix and dissolve the prescribed amount of argatroban and sodium hydroxide in 300ml of water for injection, heat the sterilized blank liposomes to 56°C and keep warm, slowly add the aqueous solution of argatroban under stirring, continue Incubate at 56° C. for 10-15 minutes, stir and mix to obtain argatroban liposome injection.
Embodiment 3
[0045] Embodiment 3: the preparation of argatroban liposome injection, specifically according to the following prescription by weight
[0046]
[0047]Injection composition and component content are according to above-mentioned (embodiment 3) prescription, osmotic pressure regulator selects mannitol for use, buffer solution selects the acetate buffer solution that pH is 7.5 for use, all the other specific preparation methods are the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com