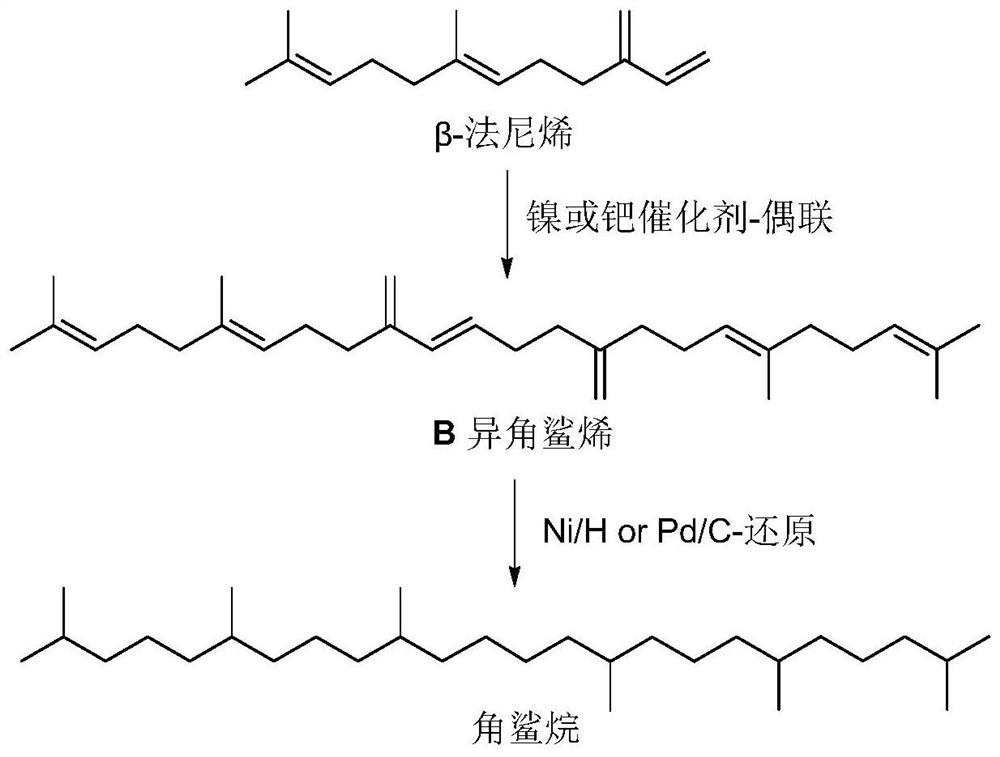
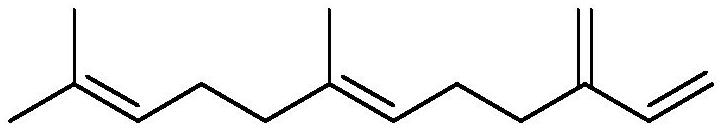
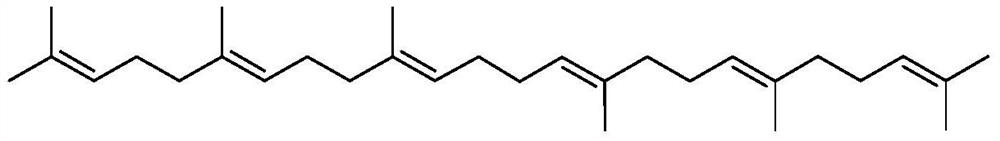
Catalytic system for farnesene coupling and squalane preparation method
A farnesene coupling and catalytic system technology, applied in chemical instruments and methods, catalytic reactions, hydrogenation hydrocarbon production, etc., can solve the problems of low yield of target products, no industrial value, cumbersome post-treatment, etc., and achieve high reliability The effects of operability, simple separation, and optimization of the reaction system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In a 50mL reaction flask, add NiCl 2 (PPh 3 ) 2 (320mg, 0.4893mmol), PPh 3 (256.7mg, 0.9787mmol), HCOONa (665.6mg, 9.7867mmol), replace the gas three times to ensure that the oxygen in the reaction bottle is excluded. N 2 Inject farnesene 6.0mL (24.4667mmol, 1mmolmL -1 ), and inject 18.52mL of isopropanol as a solvent, and replace the gas again to remove oxygen. The reaction was stirred at 70°C for 23h, and the polymerization was stopped. The reaction solution was filtered with celite, the filtrate was collected, and the solvent was drained to obtain a yellow-brown oil, weighing 4.7890 g. The GC-Mass analysis of the yellow-brown oil showed that the selectivity of squalene was 94%, and the yield was 90.0%.
Embodiment 2
[0047] In a 50mL reaction flask, add NiCl 2 (33.03mg, 0.2447mmol), PPh 3 (128.3mg, 0.4893mmol), Na 2 CO 3 (518.6mg, 4.893mmol), replace the gas three times to ensure that the oxygen in the reaction bottle is excluded. N 2 Inject farnesene 6.0mL (24.4667mmol, 1mmolmL -1 ), and inject 18.52mL tetrahydrofuran as a solvent, and replace the gas again to remove oxygen. The reaction was stirred at 85°C for 18h, and the polymerization was stopped. The reaction solution was filtered with diatomaceous earth, the filtrate was collected, and the solvent was drained to obtain a yellow-brown oil, weighing 4.1089 g. The GC-Mass analysis of the yellow-brown oil showed that the selectivity of squalene was 84%, and the yield was 60.8%.
Embodiment 3
[0049] In a 50mL reaction flask, add Pd(OAc) 2 (27.46mg, 0.1223mmol), PPh 3 (64.2mg, 0.2447mmol), Na 2 CO 3 (259.3mg, 2.4467mmol), replace the gas three times to ensure that the oxygen in the reaction bottle is excluded. N 2 Inject farnesene 6.0mL (24.4667mmol, 1mmolmL -1 ), and inject 18.52mL of isopropanol as a solvent, and replace the gas again to remove oxygen. The reaction was stirred at 60°C for 60h, and the polymerization was stopped. The reaction solution was filtered with diatomaceous earth, the filtrate was collected, and the solvent was drained to obtain a yellow-brown oil, weighing 3.9018 g. The GC-Mass analysis of the yellow-brown oil showed that the selectivity of squalene was 91%, and the yield was 71.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com