Kit for quantitatively detecting psychotropic drugs in biological sample and application thereof
A biological sample, quantitative detection technology, applied in the field of psychiatric drug detection kits, can solve the problems of prolonged detection time, unfavorable operation of clinical detection personnel, and expensive environment of isotope consumables.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: the detection method of complete mixed standard
[0075] A method for simultaneously detecting psychotropic drugs or their metabolites in the body, said method adopts ultra-high performance liquid chromatography-ultraviolet detection method, comprising the following steps:
[0076] S1 chromatographic conditions: (1) Chromatographic column: C 18 Column, ACQUITY UPLC BEH C 18 The diameter is 2.1mm*50mm, and the filler particle size is 1.7μm; (2) mobile phase: phase A is 0.05v / v% formic acid-water solution; phase B is 0.05v / v% formic acid-methanol solution; (3) detector It is an ultraviolet (UV) detector, and the detection wavelength of the ultraviolet detector is full-wavelength scanning; (4) the flow rate of the mobile phase is 0.4mL / min; (5) the column temperature is 40 ° C; (6) the injection volume of the working solution Both are 10.0μL;
[0077] The preparation of S2 working solution includes:
[0078] Preparation of S20 needle washing solution: Mea...
Embodiment 2
[0121] Embodiment 2 takes the detection of the patient serum sample of clozapine, quetiapine fumarate medicine
[0122] S1 chromatographic conditions, S22 The preparation method of mixed standard solution is the same as embodiment 1;
[0123] Preparation of S231 serum test solution: 20 μL each of the internal standard 1-metoclopramide working solution and the internal standard 2-carbamazepine working solution prepared by S21 were precisely drawn, blown dry with nitrogen and mixed with 500 μL thawed frozen serum sample to be tested Mix into a 7mL centrifuge tube, vortex for 1min for the first time, add 200μL of 2mol / L aqueous sodium hydroxide solution, and 3mL of methyl tert-butyl ether, and vortex for 3min for the second time, then put the vortexed Centrifuge the centrifuge tube at a speed of 3000 rpm / centrifuge for 5 minutes, take the centrifuged supernatant and dry it with nitrogen, add 150 μL of methanol-water solution containing 0.1v / v% formic acid and a concentration of 1...
Embodiment 3
[0126] The detection of the serum sample of the patient of embodiment 3 taking amisulpride
[0127] S1 chromatographic conditions, S22 The preparation method of mixed standard solution is the same as embodiment 1;
[0128] S231 need testing solution preparation, S3 measuring method are with embodiment 2:
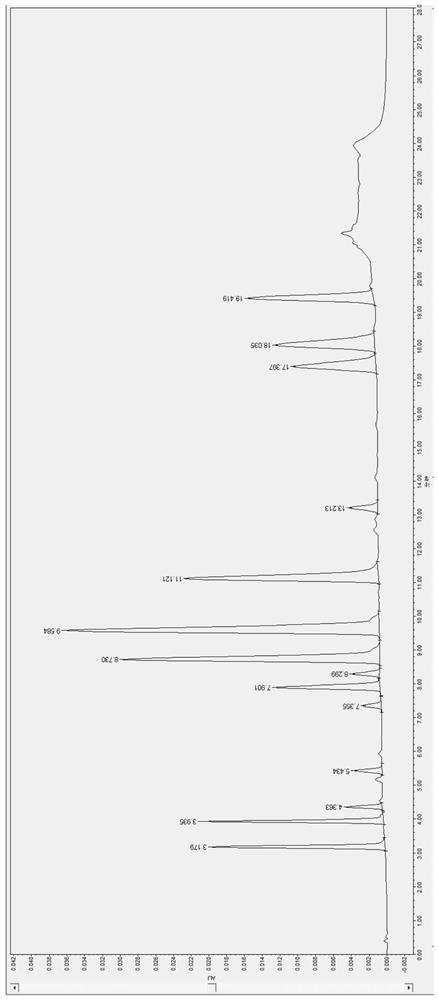
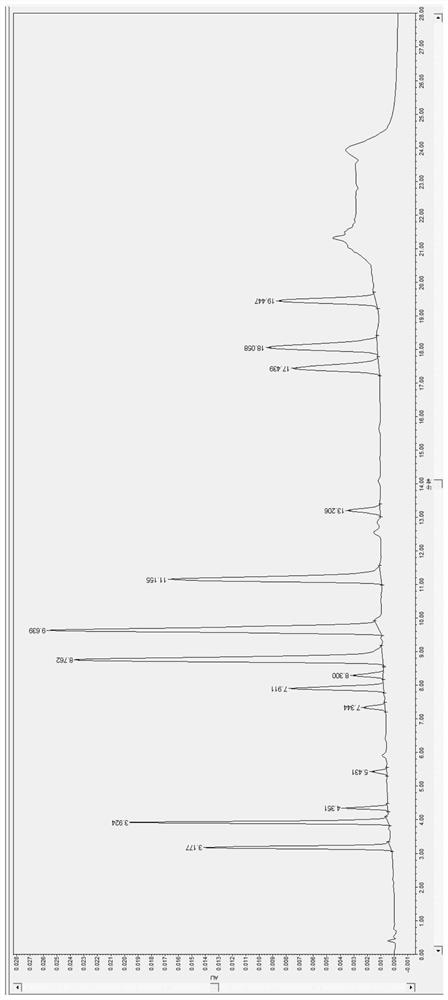
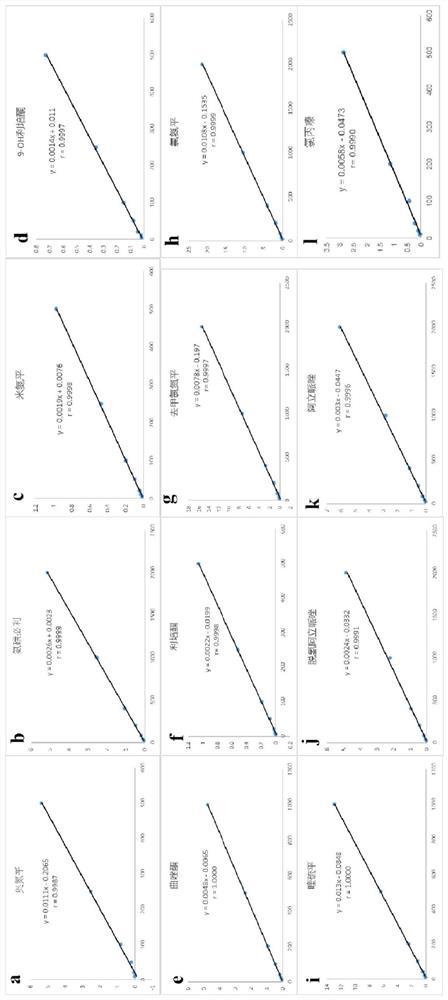
[0129] The result is as Figure 6 As shown, obtain the retention time of the psychotropic drug and its metabolites (t R ), to get final product; The retention time t of amisulpride R The internal standard 1 metoclopramide was 4.361 min and the peak area Area was 25131; the internal standard 2 carbamazepine was 13.218 min and the peak area Area was 39927. Calculate the peak area ratio of the peak area of amisulpride / internal standard 1 metoclopramide to be y, substitute into the linear equation y=0.0026x+0.0023, C=X*1 / 0.5mL, calculate the blood drug of amisulpride Concentration C was 1103.6 ng / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com