Method for synthesizing 4-(aminomethyl)benzoic acid
A technology of aminomethylbenzoic acid and bromomethylbenzoic acid, applied in the field of pharmaceutical synthesis, can solve problems such as being unsuitable for industrial production, unable to purchase benzyl cyanohalide, poor in selectivity, etc., and achieves large industrial practical value, bromination reaction The effect of high selectivity and high utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
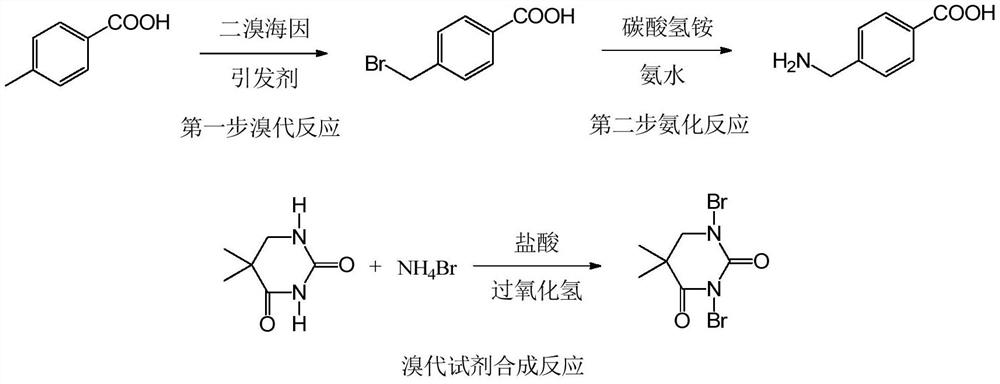
[0030] Synthesis of 4-aminomethylbenzoic acid route:
[0031]
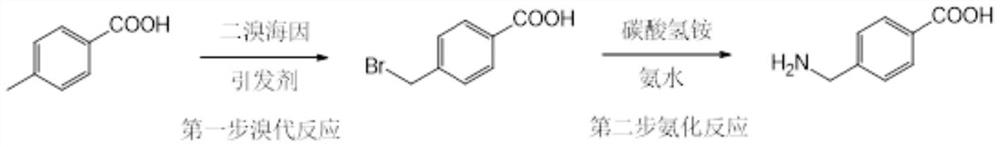
[0032] 1) Add 40.85 g of toluine to the reaction bottle, 2.96 g of azo diisobutyronitrile, 45.46g of brombide (commercial procurement), industrial dichloromethane 327ml, reflow reaction, temperature 39 ° C, reaction 9h. Covered to 30 ° C, 59 ml of purified water was added, stirred for 1 h, filtered, and the filter cake was to bromine methylbenzoic acid, with a mass of 98.9%, purity 99.89%.
[0033] It can be seen from the above steps to employ the utilization of the bromide of the present invention is greater than 95%; and the utilization rate is less than 50%, which in the prior art uses the present invention to further reduce costs.
[0034] 2) Add a carbonate 67.96 g, 21% ammonia water from 193.55 g, purified water 185 ml, and stirred at room temperature, adding 43.01 g of bromomethylbenzoic acid, heating, at 50 ° C for 1 h in step 1. Filtration, the filtrate was concentrated to viscous, filtered, and cake. The f...
Embodiment 2
[0043] 1) Add 40.85 g of tolueneic acid to the reaction bottle, 0.99 g of azo diisobutyronitrile, 43.75 g of dibromo sea (this brominated agent is used in accordance with the secondary brombide synthesis method in Example 1, with the second Zai Sea was represented by raw materials, which was more than 98.0% of the purity of more than 98.0%), 163 ml of industrial dichloromethane, reflow reaction, temperature 39 ° C, reaction 12h. Covered to 30 ° C, 59 ml of purified water was added, stirred for 1 h, filtrate, and the filter cake was to bromide methylbenzoic acid, the mass was 98.3%, and the purity was 99.78%.
[0044] 2) Add carbonate 67.96 g, 21% ammonia water from 193.55 g, purified water 185 ml, and dissolved in step 1, and 10 ° C was added to 50 ° C for 1 h. Filtration, the filtrate was concentrated to viscous, filtered, and cake. The filter cake was added 193.55 g of ammonia water, stirred at room temperature, concentrated to the material pH = 7.5, filtered, the filter cake wa...
Embodiment 3
[0049] 1) Add 40.85 g of toluine to the reaction bottle, 2.50 g of azo diisobutyronitrile, 45.46 g of dibromo sea (this brominated agent is used in accordance with the two brombide synthesis methods in Example 1, with the second Zai Sea was represented by raw materials, which was greater than 98.0% of the purity of more than 98.0%), 327 ml of industrial grade 1,2-dichloroethane, reflow reaction, temperature 83 ° C, reaction for 6 h. Covered to 30 ° C, 59 ml of purified water was added, stirred for 1 h, filtered, and the filter cake was ptchlorobenzoic acid, the mass was 97.7%, and the purity was 99.71%.
[0050] 2) Add carbonate 67.96 g, 21% ammonia water from 193.55 g, purified water 185 ml, and dissolved in step 1, and 10 ° C was added to 50 ° C for 1 h. Filtration, the filtrate was concentrated to viscous, filtered, and cake. The filter cake was added 193.55 g of ammonia water, stirred at room temperature, concentrated to the material pH = 7.5, filtered, the filter cake was hig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com